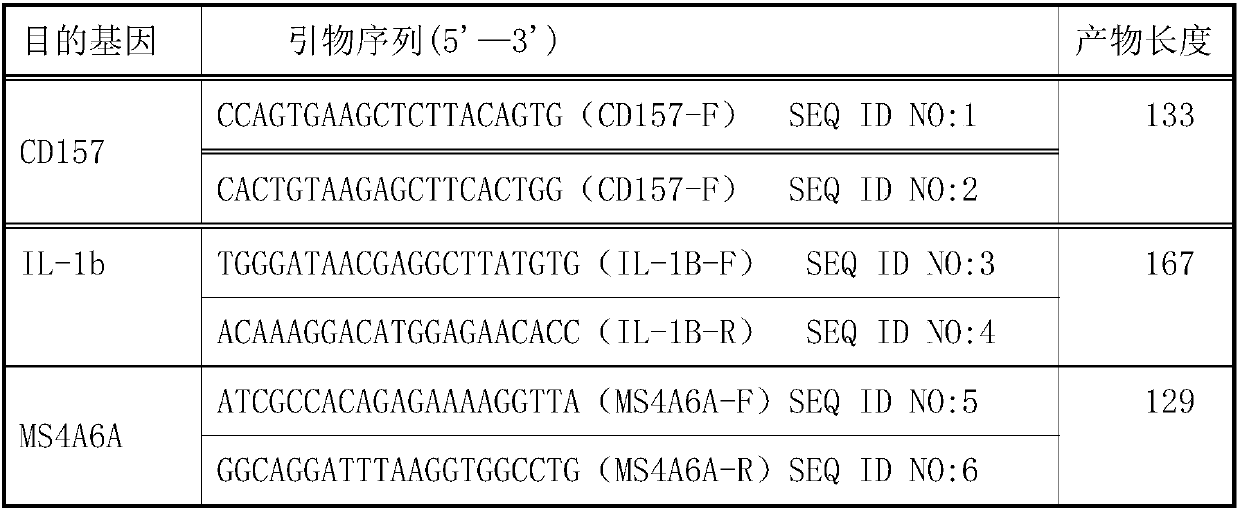
Kit and primer pair combinations for distinguishing active tuberculosis patients and non-tuberculous pneumonia patients
An active, tuberculosis technology, applied in the biological field, can solve problems such as low sensitivity, high laboratory biosafety requirements, and inability to meet clinical and tuberculosis prevention and control requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Example 1: Preparation of peripheral blood mononuclear cell (PBMC) suspension
[0023] Add 5ml of lymphocyte separation solution (Fresenius Kabi NOrge As: LYS3773) to the centrifuge tube; take 2ml of heparin anticoagulated venous blood from the above different types of people and mix the same amount of 1M phosphate buffer (PBS) thoroughly to obtain a mixed solution. Use a pipette to slowly superimpose the mixed solution on the lymphocyte separation liquid surface along the tube wall, keeping a clear interface, and centrifuge at 2000 rpm for 20 minutes; use a pipette to suck the middle cloud layer into another centrifuge tube and then add 5 Wash the cells with double volume of 1M PBS, 1500 rpm / dissociation for 10 minutes, discard the supernatant, and wash the cells again under the same conditions, then add 1ml of RPMI1640 (Thermo scientific: SH30807.01b) containing 10% calf serum volume percentage , Resuspend the cells to obtain PBMC suspension; in each case, take 20μl of P...
Embodiment 2
[0024] Example 2: RNA extraction
[0025] RNeasy Mini Kit (Cat. No. 74106) of Qiagene Company was used to extract RNA from the PBMC suspensions of the three groups of people obtained above. The specific operation is: take the above containing 1×10 6 The PBMC suspension of each cell is placed in a centrifuge tube with DNase and RNase removed at 3000 rpm / centrifugation for 10 minutes, and the supernatant is discarded; 350μl×Buffer RLT is added to the cell pellet, mixed well and lysed; 250μl absolute ethanol is added , Mix well, transfer the liquid to the RNeasy column, centrifuge at 8,000g for 30 seconds, discard the waste; add 350μl Buffer RW1 and centrifuge at 8,000g for 30 seconds, discard the waste; add 80μl DNase solution (10 μl DNase+70μlBuffer RDD) , Digest on the column for 15 minutes, centrifuge at 8,000g for 30 seconds, discard the waste; add 350μl Buffer RW1, centrifuge at 8,000g for 30 seconds, discard the waste; add 500μl Buffer RPE, centrifuge at 8,000g for 30 seconds...
Embodiment 3
[0026] Example 3: Reverse transcription
[0027] Using TAKARA's reverse transcription kit (DRR047), take 0.5μg of RNA obtained in step 2 for reverse transcription. Compared with traditional reverse transcription kits, this kit adds the steps of removing genomic DNA, which can guarantee to the greatest extent RNA purity and specificity of amplification.
[0028] The steps are as follows:
[0029] (1) Removal reaction of genomic DNA
[0030] Table 1 Removal reaction system of genomic DNA
[0031] Reagent
[0032] After preparing the reaction system according to Table 1, warm it at 42°C for 2 minutes, and the resulting RNA reaction solution from which genomic DNA has been removed is stored at 4°C.
[0033] (2) Reverse transcription reaction
[0034] The preparation of the reaction system is carried out on ice, and the specific system is as follows:
[0035] Table 2 Reverse transcription reaction system table
[0036] Reagent
[0037] After preparing the reaction system according to Tabl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com