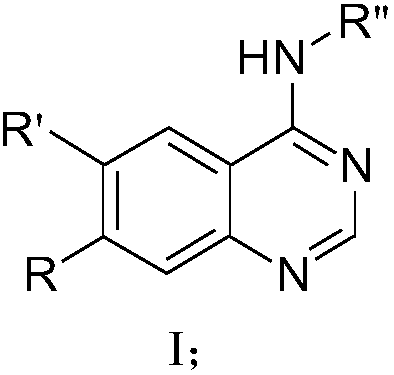
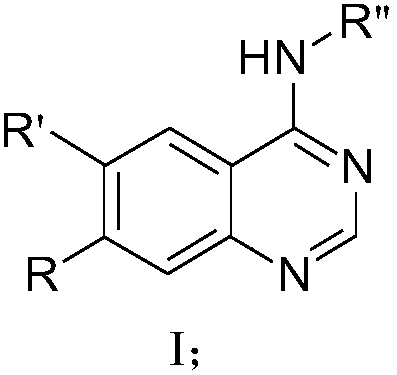
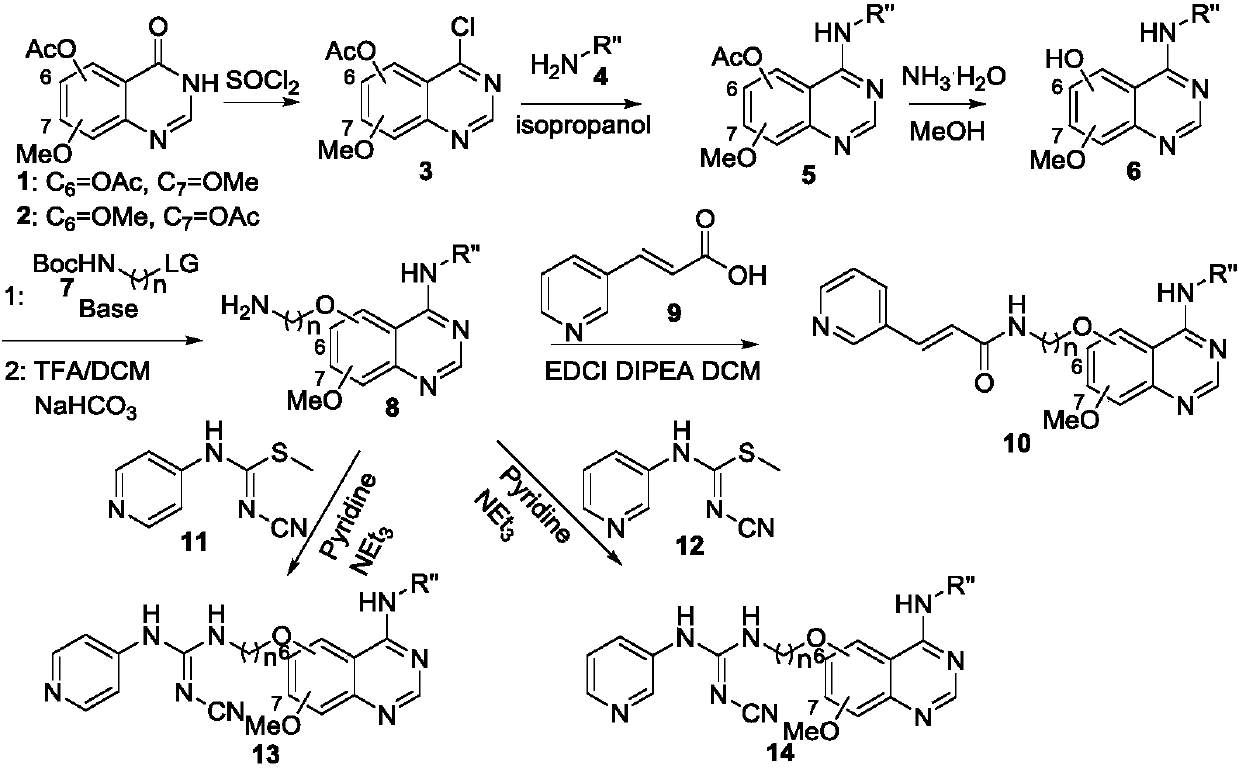
Benzo nitrogen hetero-aromatic ring compound, and preparation method and applications thereof
A technology of heteroaromatic rings and compounds, which is applied in the field of benzazeteroaromatic ring compounds and their preparation, and can solve the problems of easy tolerance and low curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0137]
[0138] Step 1, 4-chloro-7-methoxyquinazolin-6-ol acetate
[0139] Place 3,4-dihydro-7-methoxy-4-oxoquinazolin-6-ol acetate (4g, 17.1mmol) in a 100mL conical flask, add 40mL dichlorohydrin at room temperature After sulfone and 1 drop of DMF, heat and reflux for 24 hours; distill off most of the thionyl chloride, add ice water, filter, and dry to obtain 4-chloro-7-methoxyquinazolin-6-alcohol acetate ( 4.07 g), yield 94%.
[0140] 1 H-NMR (400MHz, CDCl 3 ): δ8.95(s,1H),7.90(s,1H),7.44(s,1H),4.02(s,3H),2.39(s,3H).
[0141] Step 2, 4-((3-ethynylphenyl)amino)-7-methoxyquinazolin-6-ol acetate
[0142] 4-Chloro-7-methoxyquinazolin-6-ol acetate (4.07g, 16.1mmol) and 3-aminophenylacetylene (3.77g, 21.3mmol) were placed in 140mL of isopropanol, heated to reflux for 12 hours, cooled, and filtered to obtain 4-((3-ethynylphenyl)amino)-7-methoxyquinazolin-6-ol acetate (4.59 g), with a yield of 85%.
[0143] 1 H-NMR (400MHz, DMSO-d 6): δ11.48(s,1H),8.94(s,1H),8.80(s,1H),7....
Embodiment 2
[0154]
[0155] In step 4 of Example 1, 2-(tert-butoxycarbonylamino)ethyl iodide was replaced by 3-(tert-butoxycarbonylamino)propyl bromide, and the others were the same as in Example 1 to obtain compound 1-2, (E )-N-(3-(4-(3-ethynylphenylamino)-7-methoxyquinazolin-6-oxyl)propyl)-3-(pyridine-3-substituted)acrylamide.
[0156] MS(EI,m / z):480(M + +1). 1 H-NMR (400MHz, CDCl 3 ): δ8.62(s,1H),8.59(s,2H),8.51(d,J=4.7Hz,1H),7.93(s,1H),7.83(d,J=8.0Hz,1H),7.61 (d,J=8.0Hz,1H),7.51(d,J=15.7Hz,1H),7.47(s,1H),7.34–7.18(m,4H),7.14(s,1H),6.52(d, J=15.7Hz, 1H), 4.09(t, J=6.1Hz, 2H), 3.91(s, 3H), 3.60(q, J=6.0Hz, 2H), 3.02(s, 1H), 2.11–2.05( m,6.0Hz,2H). 13 C NMR (125MHz, CDCl 3 ) Δ165.8,156.7,154.9,153.6,150.4,148.9,147.4,139.1,137.6,130.5,127.7, 125.5, 122.7, 122.7.3,7.3,13.2.3.2.7.3.7, 103.2.3.7,13.2.3.7.6,13.3.2. ,56.1,37.5,28.6.
Embodiment 3
[0158]
[0159] In step 4 of Example 1, 2-(tert-butoxycarbonylamino) ethyl iodide was replaced by 4-(tert-butoxycarbonylamino) butyl bromide, and the others were the same as in Example 1 to obtain compound 1-3, (E )-N-(4-(4-(3-ethynylphenylamino)-7-methoxyquinazolin-6-oxyl)butyl)-3-(pyridine-3-substituted)acrylamide.
[0160] MS(EI,m / z):494(M + +1). 1 H-NMR (400MHz, CDCl 3 ): δ8.60(s,1H),8.56(s,2H),8.49(d,J=4.7Hz,1H),7.91(s,1H),7.81(d,J=8.0Hz,1H),7.60 (d,J=8.0Hz,1H),7.49(d,J=15.7Hz,1H),7.45(s,1H),7.31–7.15(m,4H),7.12(s,1H),6.50(d, J=15.7Hz, 1H), 4.06(t, J=6.1Hz, 2H), 3.88(s, 3H), 3.60(q, J=6.0Hz, 2H), 3.02(s, 1H), 2.13–2.07( m,4H). 13 C NMR (125MHz, CDCl 3 )δ165.3, 156.3, 154.6, 153.3, 150.1, 148.6, 148.0, 147.2, 139.0, 137.3, 134.2, 130.3, 128.7, 127.5, 125.3, 123.5, 122.7, 122.6, 109.2, 107.5, 103.2 ,37.6,29.7,28.7.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com