Prosthetic linking arm, its synthesis method and the synthesis method of diubiquitin
A synthesis method and linker technology, which is applied in the field of prosthetic linker, its synthesis method and the synthesis of diubiquitin, can solve the problems of large prosthetic group hindrance, harsh removal conditions, large steric hindrance, etc. In addition to the effects of mild conditions, high chemical connection efficiency, and avoidance of non-denaturing conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0045] The embodiment of the present invention discloses a synthesis method of a prosthetic linking arm having a structure of formula (I) applied to the preparation of diubiquitin, comprising the following steps:
[0046] A), reacting the compound having the structure of formula (II) and the compound having the structure of formula (III) under the catalysis of a basic compound to obtain the compound having the structure of formula (IV);
[0047] Under the action of a catalyst, the compound with the structure of formula (IV) is reacted in methanol to obtain the compound with the structure of formula (V);
[0048] reacting N-hydroxyphthalimide with a compound of formula (VI) under the action of a catalyst to obtain a compound of formula (VII);
[0049] B), under the action of a catalyst, the compound having the structure of formula (V) is reacted with the compound having the structure of formula (VII) to obtain the compound having the structure of formula (VIII);
[0050] C) reac...
Embodiment 1
[0079] Embodiment 1: Synthesis of novel prosthetic group linking arm O-(2-((2-nitrobenzyl) mercapto) ethoxy) hydroxylamine
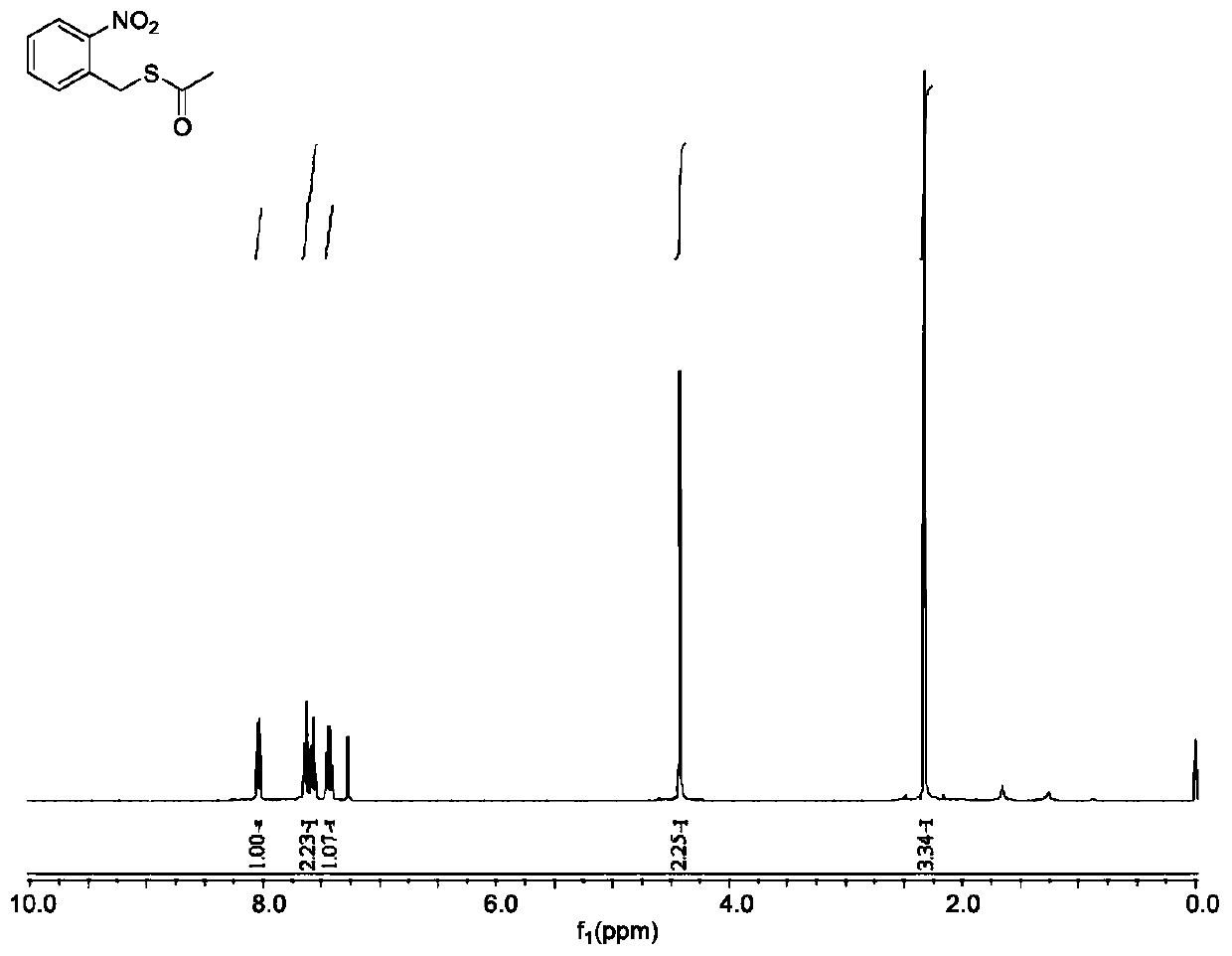
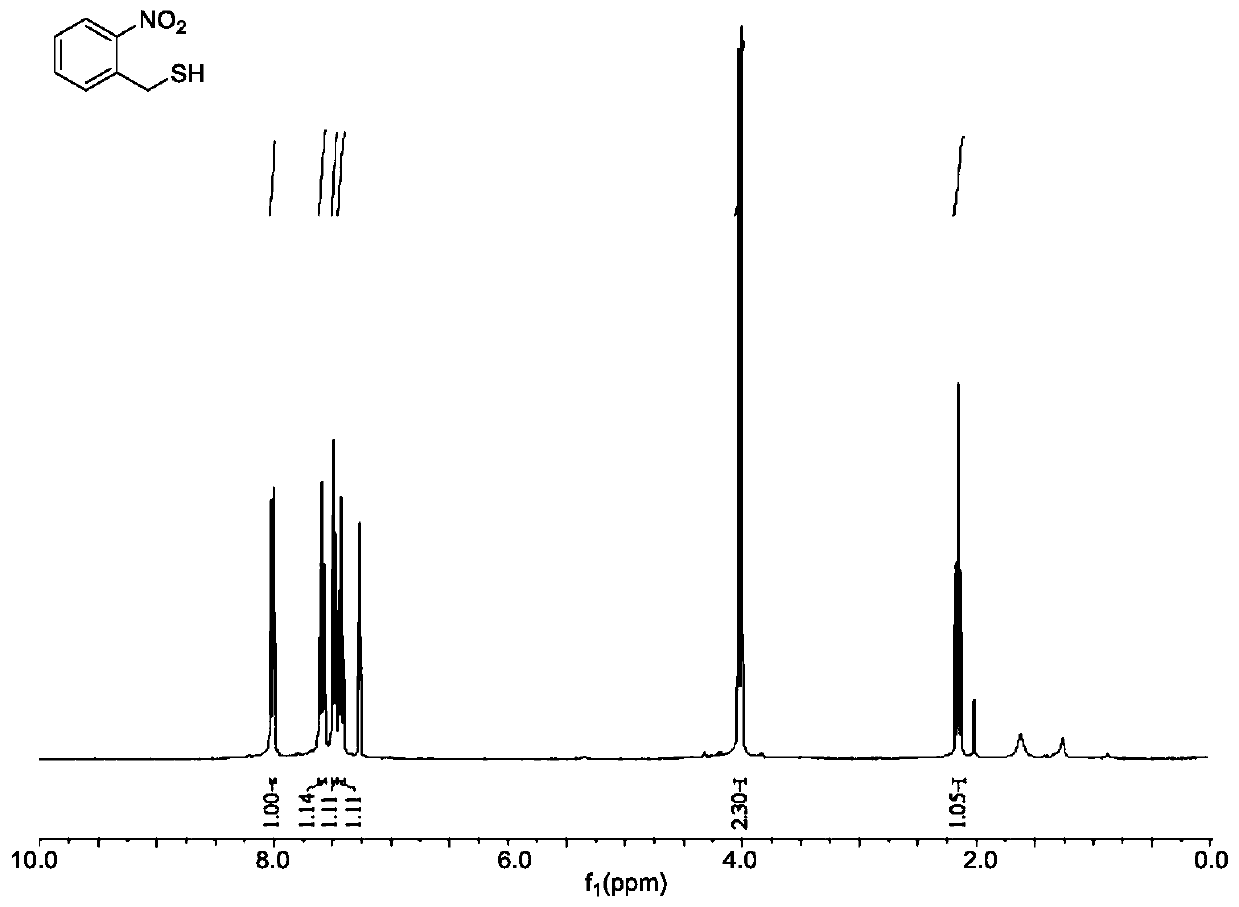
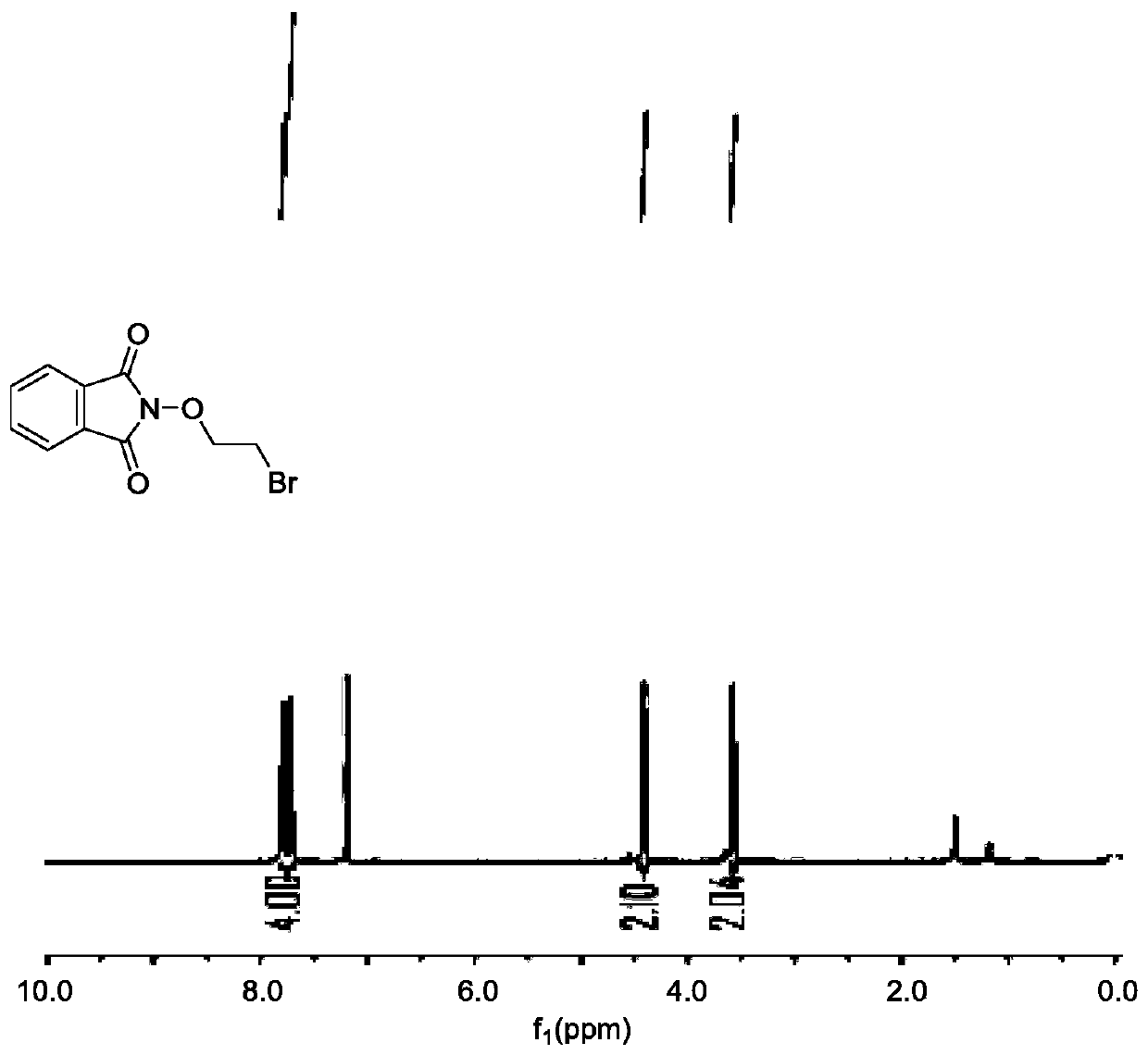
[0080] Compound IV1: Weigh 4.04g of o-nitrobenzyl chloride (23.6mmol) and 3.86g of potassium carbonate and place them in a round-bottomed flask respectively, add 24ml and 48ml of tetrahydrofuran solution for dissolution, and add 2ml of thioacetic acid (30.5mmol); after 30 minutes, the tetrahydrofuran solution containing o-nitrobenzyl chloride was added to the above solution for reaction, and the reaction progress was monitored by TLC. Evaporator to remove volatile tetrahydrofuran, dissolve the solid with an appropriate amount of dichloromethane, then extract twice with saturated sodium bicarbonate solution and water, collect the organic phase, dry with anhydrous sodium sulfate, filter, spin dry, and weigh the compound IV1 o-nitrobenzyl thioacetate 5.87g (yellow liquid), directly proceed to the next reaction. Such as figure 1 as shown, figure 1 The pro...
Embodiment 2
[0088] The preparation method is the same as in Example 1, except that in the step of preparing compound IV1, the solvent is water and acetonitrile with a volume ratio of 2:1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com