Ligand molecular compound with diversified steric configuration and Pi electron density and application thereof
A technology of molecular compounds and ligand molecules, applied in organic chemistry, chemical instruments and methods, nanotechnology for materials and surface science, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
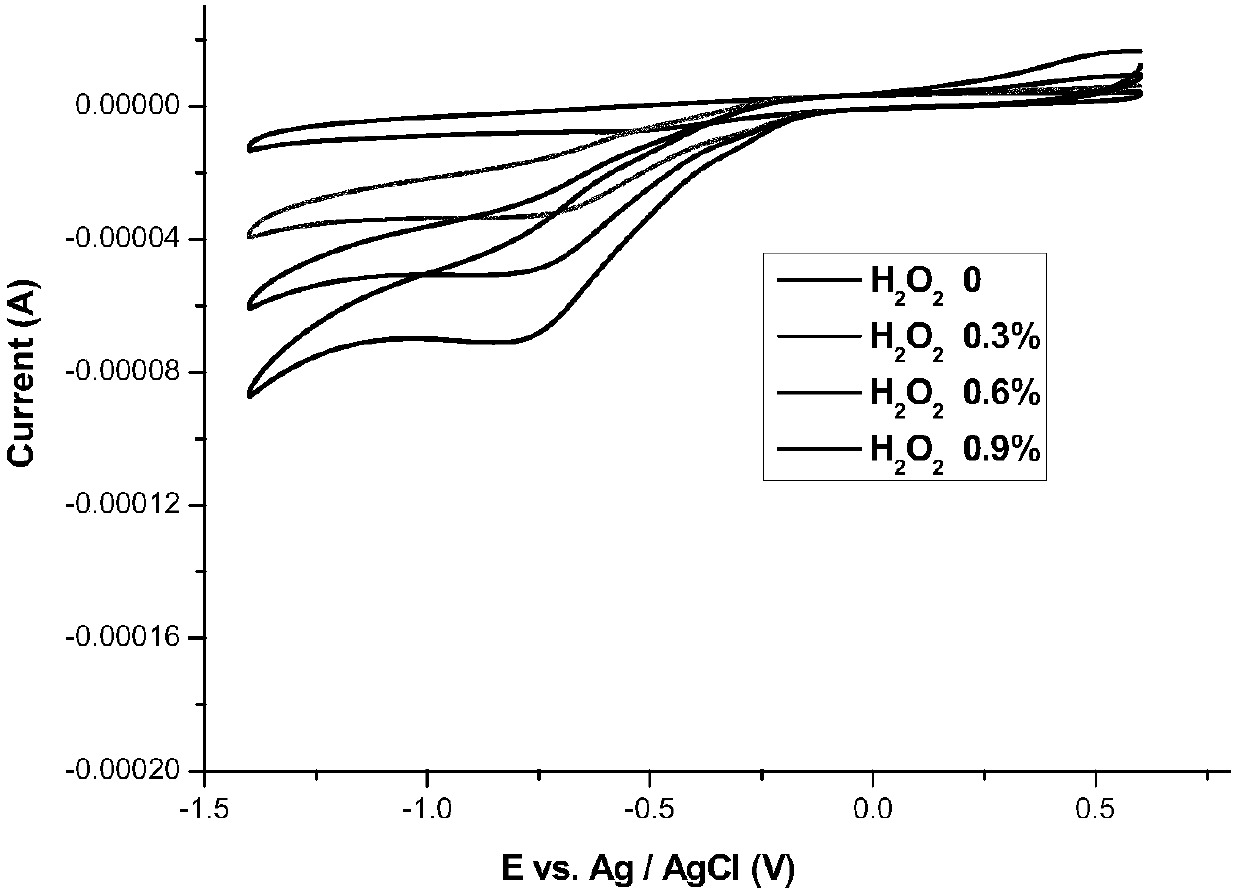
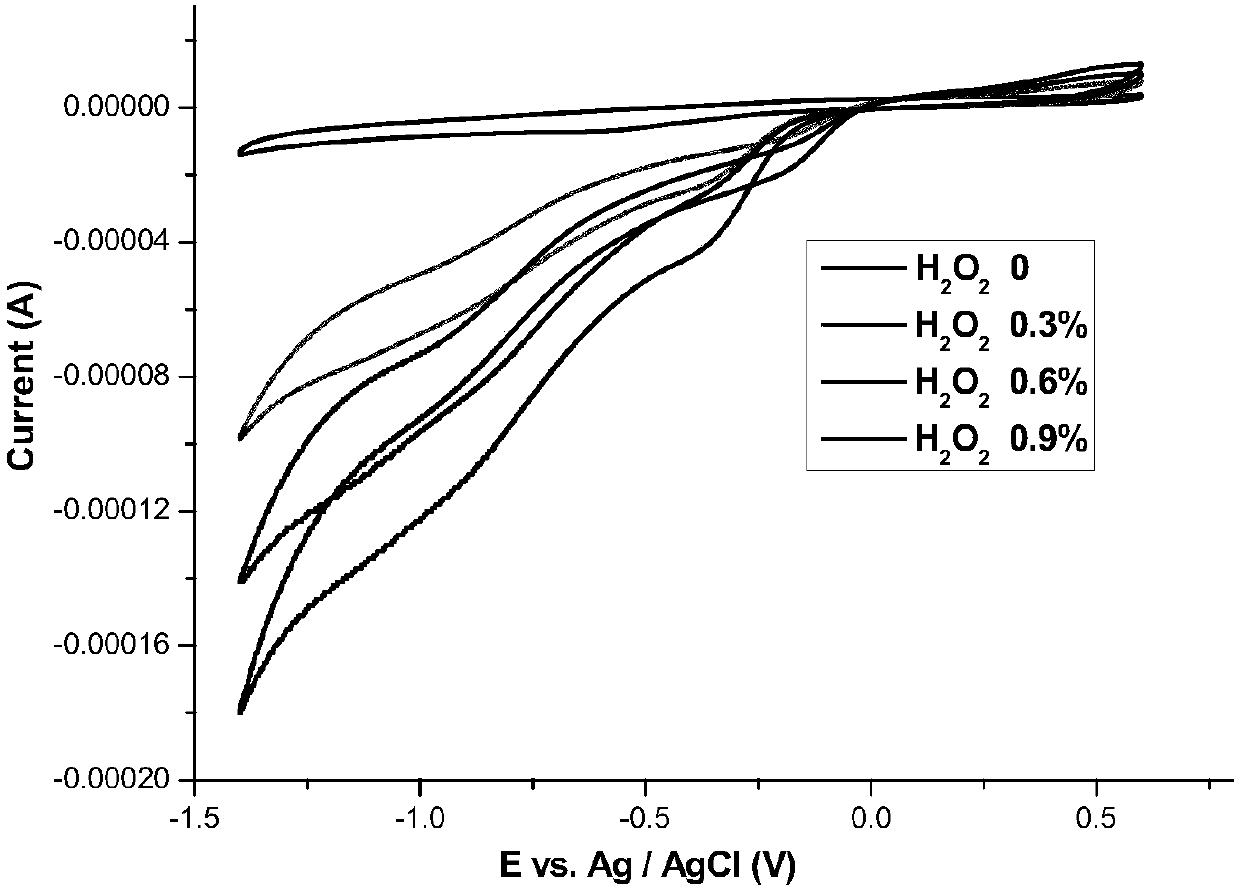
Image
Examples
Embodiment 1
[0050] Synthesis of Compound Ligand1
[0051] with R 1 =1,4-disubstituted cyclohexyl, R 2 =Phenyl, synthesize the ligand compound Ligand1.
[0052] The reaction pathway is as follows:
[0053]
[0054] Under ice-cooling, mix 11.5g (0.05mol) of ferrocenecarboxylic acid with 100mL of dichloromethane (DCM), and stir evenly. Under strong stirring, 7.0g (0.06mol) N-hydroxysuccinimide (NHS), 11.5g (0.06mol) 1-(3-dimethylaminopropyl)- 3-Ethylcarbodiimide hydrochloride (EDC·HCl). Under ice bath, react for 4-6 hours, the solution gradually becomes clear, and monitor the reaction by TLC. After the reaction was finished, it was suction filtered to obtain a dichloromethane solution of the intermediate (1), which was then used for the next step of reaction.
[0055] Under an ice bath, 8.5 g (0.075 mol) of triethylenediamine was mixed with 50 mL of dichloromethane (DCM) to dissolve, and stirred evenly. Add 24.6mL of 1,4-cyclohexanediamine until the reaction system is uniform and s...
Embodiment 2
[0060] Synthesis of Compound Ligand2
[0061] The preparation method of compound Ligand2 is similar to the preparation of compound Ligand1 in Example 1, the difference is that the 24.6mL 1,4-cyclohexanediamine used in Example 1 is replaced by 27.5mL1,6-hexanediamine, and the compound Ligand2 It is a light yellow powder with a yield of 45.7%;
[0062] 1 H NMR (500MHz, DMSO-d 6 )δ(ppm)=8.45(s,1H), 8.03(m,2H), 7.96~7.80(m,4H), 6.83~6.74(m,3H), 4.53(m,1H), 3.83(s,6H ), 2.6~2.52(m,5H), 3.21~2.83(m,6H), 2.13~1.25(m,14H), C 31 h 42 N 3 o 5 S 2 , ESI-MS: m / z 732.3 (M+1) + .
Embodiment 3
[0064] Synthesis of Compound Ligand3
[0065] The preparation method of compound Ligand3 is similar to the preparation of compound Ligand1 in Example 1, the difference is that the 0.50mL benzaldehyde used in Example 1 is replaced by 0.64mL 4-N,N-dimethylaminobenzaldehyde, compound Ligand3 It is a light yellow powder with a yield of 37.8%;
[0066] 1 H NMR (500MHz, DMSO-d 6 )δ (ppm) = 8.03 (m, 4H), 6.83 ~ 6.74 (m, 3H), 4.53 (m, 1H), 3.83 (s, 6H), 2.6 ~ 2.52 (m, 5H), 3.21 ~ 2.83 (m ,6H), 2.38~1.25(m,24H), C 31 h 49 N 3 o 5 S 2 , ESI-MS: m / z773.3 (M+1) + .
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com