Preparation method of voriconazole intermediate
A technology for voriconazole and intermediates, which is applied in the field of drug synthesis, can solve the problems of difficult realization, many reaction impurities and a large amount, and achieves the effects of low production equipment requirements, simple synthesis conditions and high total yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
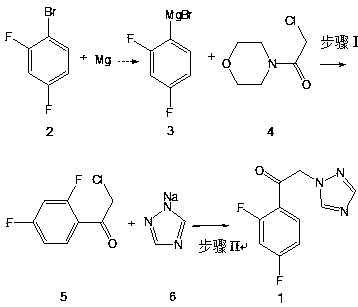
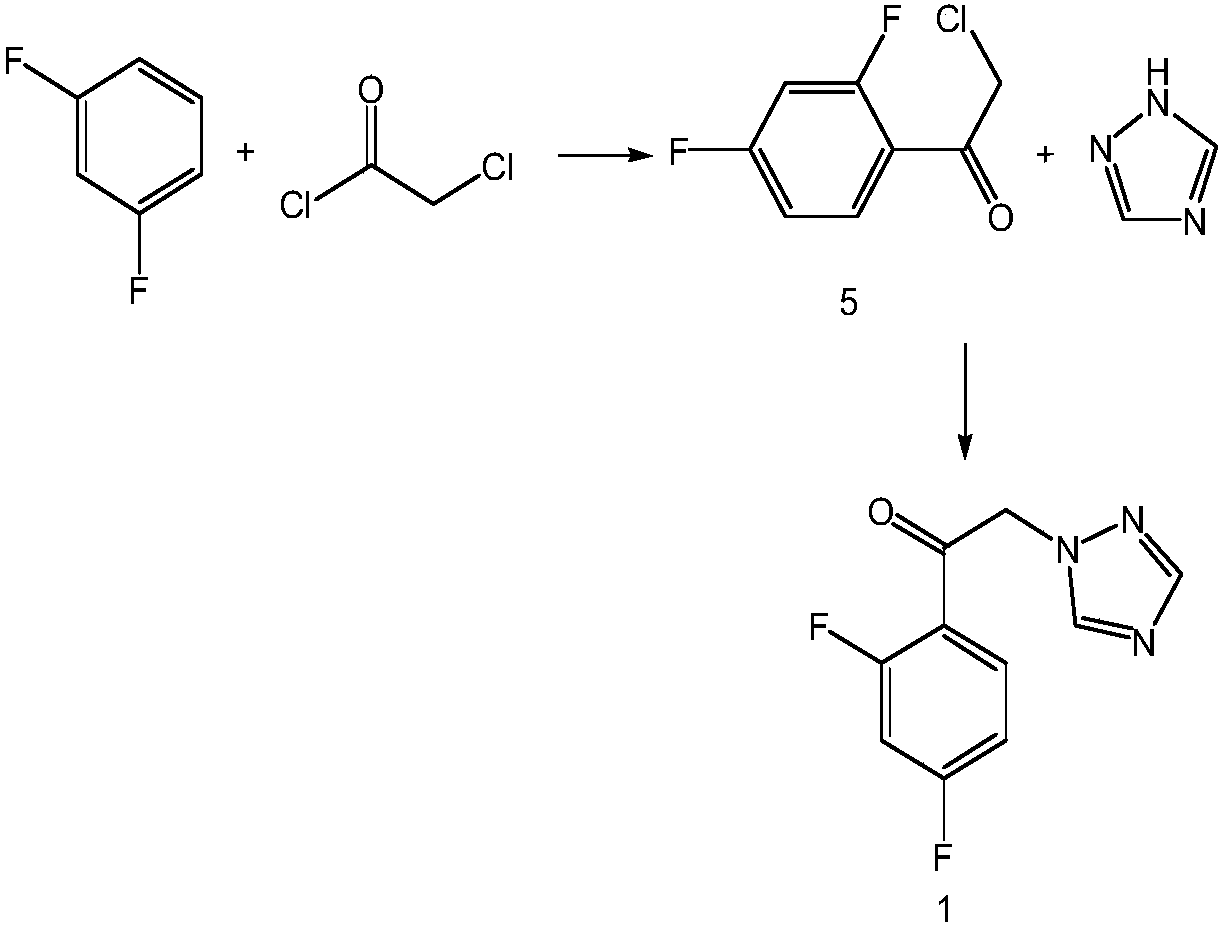
[0027] Example 1 Preparation of 2'-chloro-2,4-difluoroacetophenone (compound 5)
[0028] Prepare a dry and clean 1000mL glass four-neck bottle, equipped with a suitable mechanical stirrer, condenser tube and thermometer, pay attention to check the airtightness before adding materials, N 2 Add 96.5g of 2,4-difluorobromobenzene (0.5mol, 1.0eq), 25.5g (0.525mol, 1.05eq) of magnesium strips, 500mL of tetrahydrofuran into the reaction flask under protection, start stirring, and raise the temperature of the reaction solution to 30 ~35°C, stirred and reacted for 2h. After the reaction was completed, the reaction solution was slowly added dropwise to 90.0g (0.55mol, 1.1eq.) of 4-(2-chloroacetyl)morpholine (compound 4) tetrahydrofuran solution (90.0 g dissolved in 360ml of tetrahydrofuran), the temperature was controlled at 0°C during the dropping process, and added dropwise (about 0.05mL per drop, the same below). After the reaction finished, the reaction system was dripped with 100m...
Embodiment 2
[0029] Example 2 Preparation of 2'-chloro-2,4-difluoroacetophenone (compound 5)
[0030] Prepare a dry and clean 1000mL glass four-neck bottle, equipped with a suitable mechanical stirrer, condenser tube and thermometer, pay attention to check the airtightness before adding materials, N 2 Add 96.5g of 2,4-difluorobromobenzene (0.5mol, 1.0eq), 25.5g (0.525mol, 1.05eq) of magnesium strips, 500mL of tetrahydrofuran into the reaction flask under protection, start stirring, and raise the temperature of the reaction solution to 30 ~35°C, stirred and reacted for 2h. After the reaction was completed, the reaction solution was slowly added dropwise to 90.0g (0.55mol, 1.1eq.) of 4-(2-chloroacetyl)morpholine (compound 4) tetrahydrofuran solution (90.0 g was dissolved in 360ml of tetrahydrofuran), the temperature was controlled at 20°C during the dropwise addition, and after the dropwise addition was completed, the temperature was kept at 20°C and stirred for 4 hours. After the reaction ...
Embodiment 3
[0031] Example 3 Preparation of 2'-chloro-2,4-difluoroacetophenone (compound 5)
[0032] Prepare a dry and clean 1000mL glass four-neck bottle, equipped with a suitable mechanical stirrer, condenser tube and thermometer, pay attention to check the airtightness before adding materials, N 2 Add 96.5g of 2,4-difluorobromobenzene (0.5mol, 1.0eq), 25.5g (0.525mol, 1.05eq) of magnesium strips, 500mL of tetrahydrofuran into the reaction flask under protection, start stirring, and raise the temperature of the reaction solution to 30 ~35°C, stirred and reacted for 2h. After the reaction was completed, the reaction solution was slowly added dropwise to 90.0g (0.55mol, 1.1eq.) of 4-(2-chloroacetyl)morpholine (compound 4) tetrahydrofuran solution (90.0 g was dissolved in 360ml of tetrahydrofuran), the temperature was controlled at 40°C during the dropwise addition, and after the dropwise addition was completed, the temperature was kept at 40°C and stirred for 2 hours. After the reaction ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com