Method for preparing transition metal oxide positive electrode material of lithium
A technology of transition metal and positive electrode material is applied in the field of preparing lithium transition metal oxide positive electrode material to achieve the effects of improving electrochemical performance, easy control of reaction conditions and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] (1) Dissolve 0.45mmol3,4,9,10-perylenetetracarboxylic anhydride in 14mL of 0.128M NaOH solution, add 0.2mmol Ni(Ac) 2 2H 2 O and 0.7mmol Mn(Ac) 2 2H 2 O was dissolved in 25 mL of water. Slowly add the perylene anhydride solution dropwise into the nickel-manganese mixed solution under stirring condition, stir at room temperature for 0.5h, transfer the prepared mother liquor into a reaction kettle, and hydrothermally crystallize at 100°C for 24h;
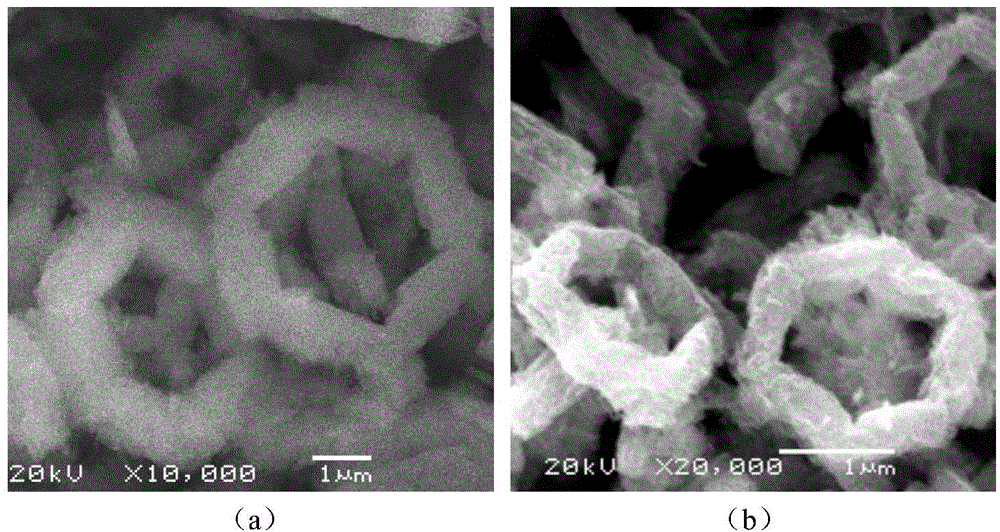
[0045] (2) After centrifugation, washing, and drying, an orange-yellow precursor was obtained. Under the JSM-6360 scanning electron microscope, as attached figure 1 (a) The nickel-manganese-organic ligand polymer shown has a six-membered ring morphology, with each side width of about 700 nm and length of 2.5 μm. The precursor was calcined in an air atmosphere at 550° C. for 1 h to obtain nickel manganese oxide. as attached figure 1 As shown in (b), the nickel manganese oxide continues the six-membered ring morphology of t...
Embodiment 2
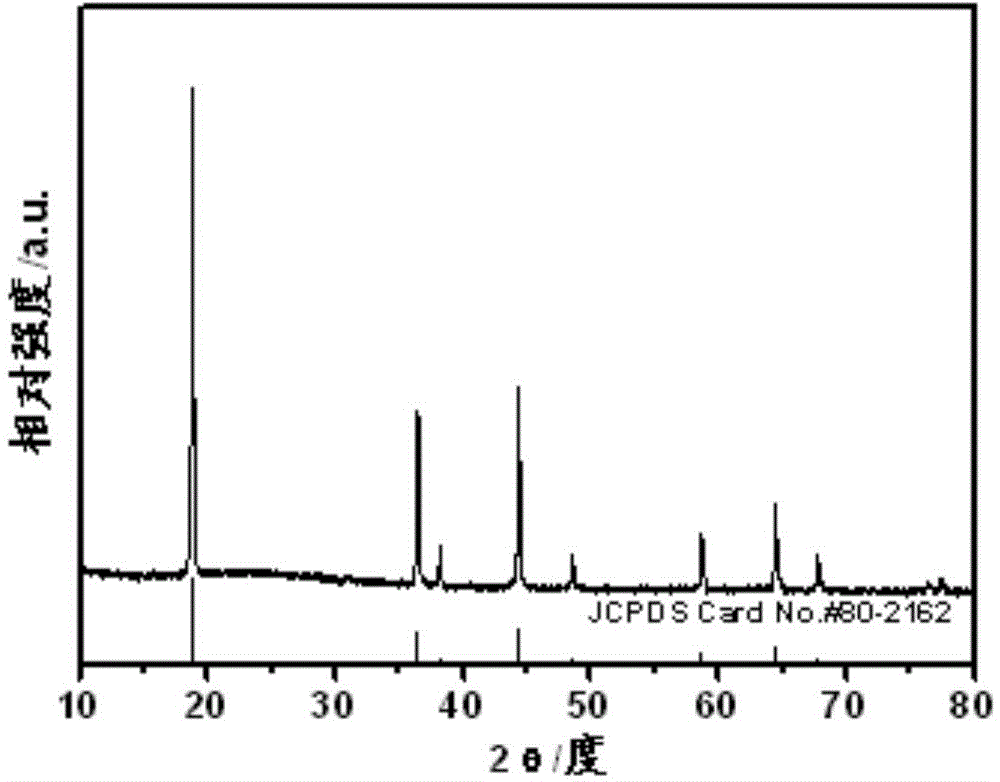
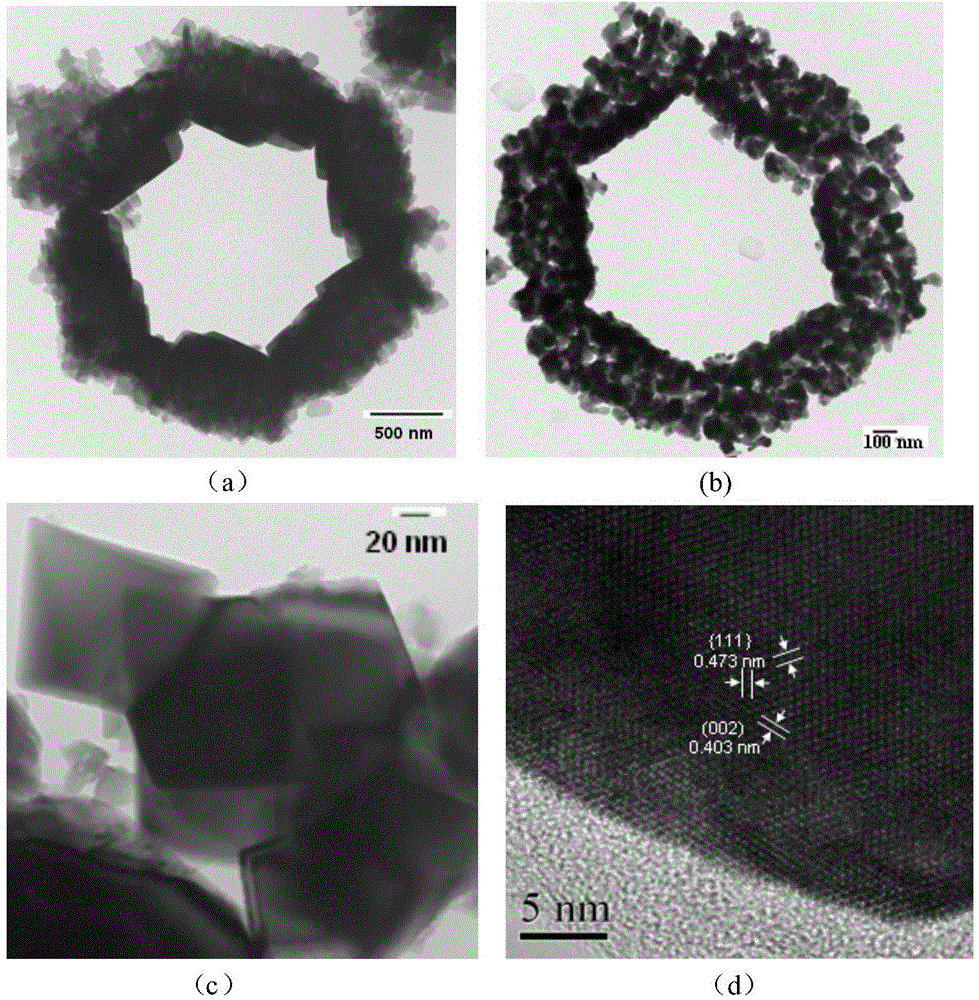
[0052] Using the preparation process of Example 1, the difference is that an excess of 15.0% Li(Ac) 2H is used 2 O was ball milled with nickel manganese oxide. X-ray diffraction spectra and transmission electron microscopy photos show that the prepared Li 1.07 Ni 0.5 mn 1.5 o 3.98 It has a spinel structure and has a nanosheet morphology of about 60nm*80nm. Using the same pole piece fabrication method as in Example 1, the material exhibits excellent cycle stability and rate performance. as attached Figure 5 As shown, when discharged at 10C, the specific capacity can reach 117mAh / g, and the capacity can still maintain 74% after 1000 cycles; when discharged at 40C, the capacity can still reach 98.0mAh / g after 500 cycles. in the attached Image 6 Among them, when charging and discharging at 1C at 55°C, the specific capacity can reach 131mAh / g, and the capacity retention rate after 350 cycles is 79.0%.
Embodiment 3
[0054] (1) Dissolve 0.45mmol phenolic resin in 14mL solution (ethylene glycol, water volume ratio is 1:1), and 0.18mmol Ni(Ac) 2 4H 2 O, 0.10mmol Co(NO 3 ) 2 ·6H 2 O and 0.70 mmol Mn(Ac) 2 4H 2 O was dissolved in 30 mL of water;
[0055] (2) Slowly add the solution of phenolic resin dropwise to the nickel-manganese mixed solution under stirring; transfer the prepared mother liquor into the reaction kettle, and crystallize it by solvent heat at 80°C for 72h;
[0056] (3) After centrifugation, washing, and drying, the precursor was roasted in an air atmosphere at 400°C for 6 hours to obtain metal oxides;
[0057] (4) With LiNi 0.5 mn 1.5 o 4 For the target product, the metal oxide was mixed with an excess of 10% lithium source (LiAc·2H 2 O, LiNO 3 The molar ratio is 1:3) dispersed in acetone, the ball-to-material ratio is 15:1, and ball milled for 2 hours;
[0058](5) The ball-milled mixture was calcined at 700 °C for 24 h, and the heating rate was 5 °C / min to obtain...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific capacity | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com