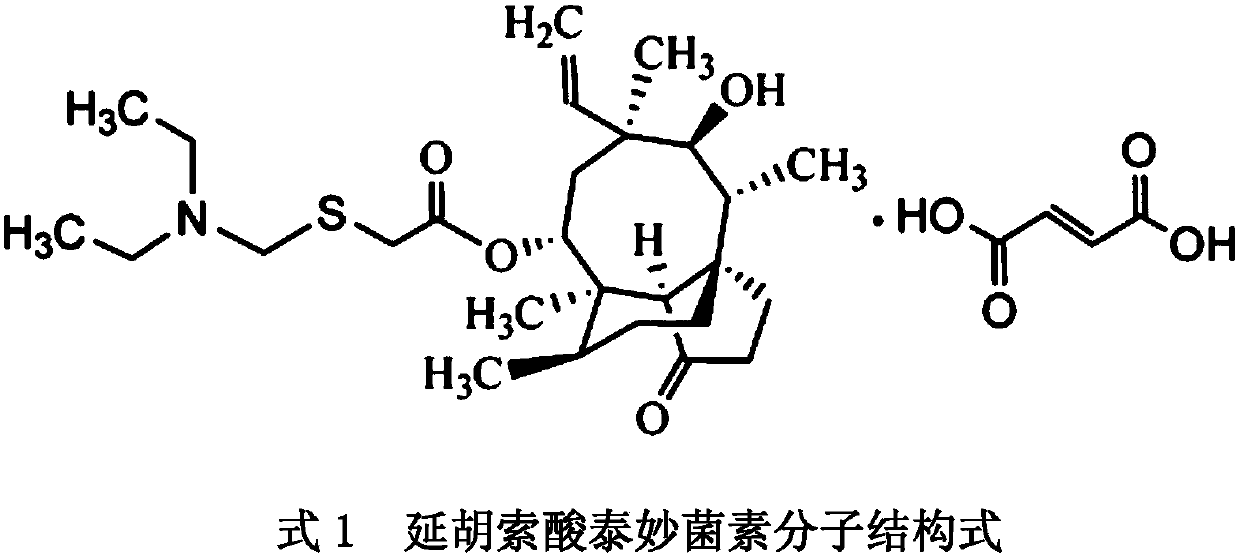
Method for preparing tiamulin
A technology of tiamulin and pleuromutilin, which is applied in the fields of thioether preparation and organic chemistry, can solve the problems of toxicity, strong pungent odor, and restriction of the market scale of tiamulin fumarate, and achieve mild conditions and low cost. Inexpensive, environmentally friendly effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
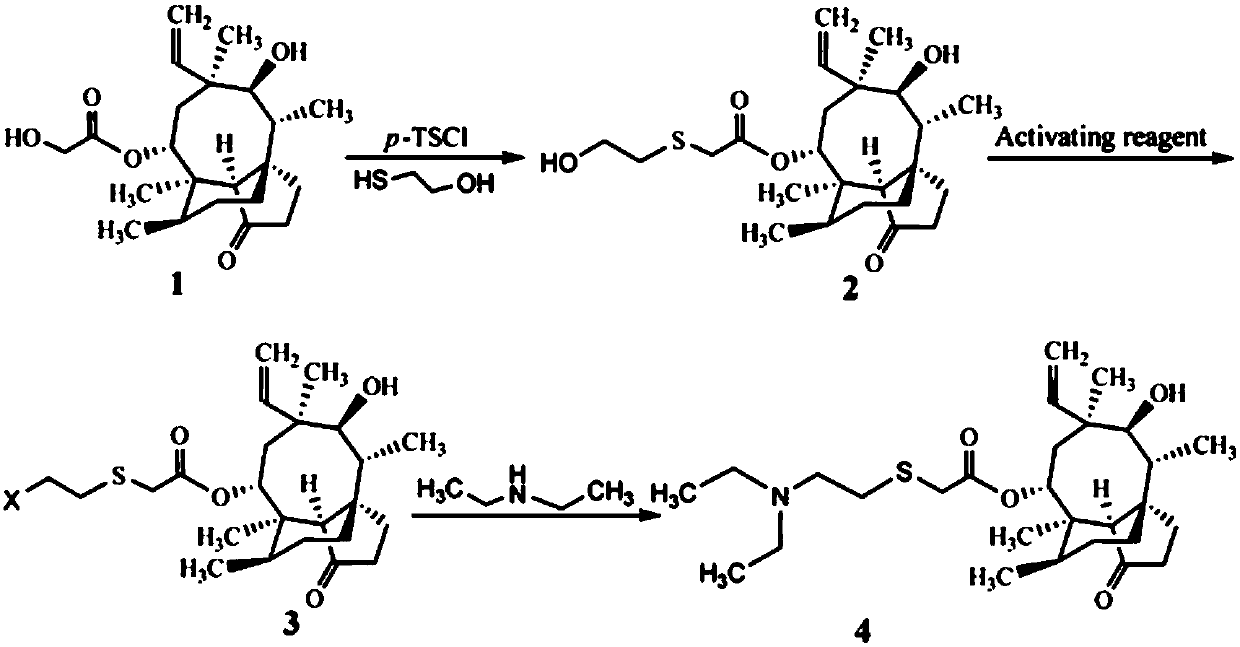
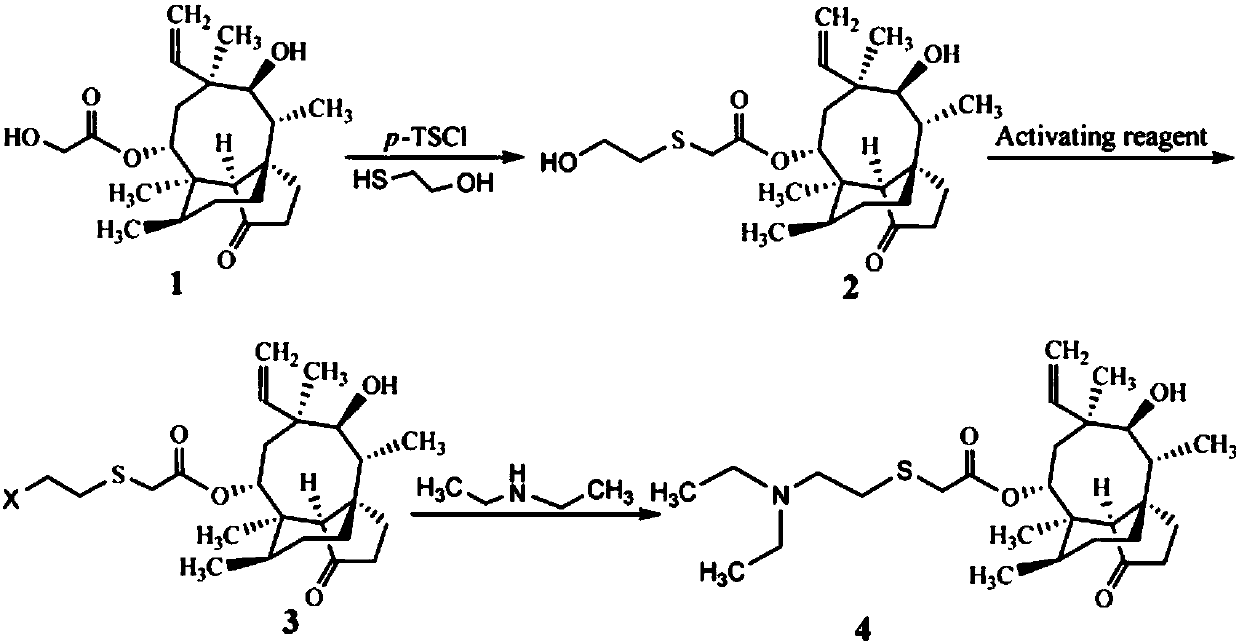
[0023] A preparation method of tiamulin, the steps are as follows:
[0024] (1) Synthesis of 14-O-[(1-hydroxypropan-2-yl)mercaptoyl]mpirin (intermediate of formula 2)
[0025] Add 8.90g (20mmol) of pleuromutilin with a purity of 85%, 4.28g (22mmol) of 98% p-toluenesulfonyl chloride and 80mL of methyl isobutyl ketone into a 250mL three-necked flask, and heat up , stirred to dissolve pleuromutilin and p-toluenesulfonyl chloride, and when the temperature rose to 60°C, 4.0 mL of NaOH aqueous solution with a concentration of 10 mol / L was added dropwise and reacted for 30 min; then 1.72 g (22 mmol) of mercaptoethanol and 20 mL of Methanol, the reaction was continued at 60°C for 3h. After the reaction, it was extracted three times with 25 mL of distilled water, and the organic phase was washed with anhydrous Na 2 SO 4 After drying and evaporating methyl isobutyl ketone to dryness, 7.55 g (yield 89%) of viscous 14-O-[(1-hydroxypropan-2-yl)mercaptoyl]mylline was obtained.
[0026] ...
Embodiment 2
[0030] A preparation method of tiamulin, the steps are as follows:
[0031] (1) Synthesis of 14-O-[(1-hydroxypropan-2-yl)mercaptoyl]mpirin (intermediate of formula 2)
[0032] Add 8.90g (20mmol) of pleuromutilin with a purity of 85%, 4.62g (24mmol) of p-toluenesulfonyl chloride and 80mL of methyl isobutyl ketone into a 250mL three-necked flask, heat and stir to make pleuromutilin Pleuromutilin and p-toluenesulfonyl chloride were dissolved, and when the temperature rose to 60°C, 4.0 mL of NaOH aqueous solution with a concentration of 10 mol / L was added dropwise and reacted for 30 min; then a mixed solution (1.57 g The mercaptoethanol was dissolved in 20 mL of methanol, and 2 mL of NaOH with a concentration of 10 mol / L was added to react for 30 min), and the reaction was continued at 60° C. for 2.5 h. After the reaction, it was extracted three times with 25 mL of distilled water, and the organic phase was washed with anhydrous Na 2 SO 4 After drying and evaporating methyl iso...
Embodiment 3
[0036] A preparation method of tiamulin, the steps are as follows:
[0037] (1) The synthesis of 14-O-[(1-hydroxypropan-2-yl)mercaptoyl]mpirin (intermediate of Formula 2) is the same as in Example 2.
[0038] (2) Synthesis of Tiamulin (Formula 4 Compound)
[0039] The intermediate 14-O-[(1-hydroxypropan-2-yl) mercaptoyl] Mutilin (compound of formula 2) synthesized by 4.24g (10mmol) is added in the methyl tert-butyl ether of 20mL, drops to After 0°C, 20 mL of a methyl tert-butyl ether solution containing 0.90 g (3.33 mmol) of PBr3 was added dropwise, and after the addition was completed, the temperature was raised to room temperature, and the reaction was stirred for 12 h. After the reaction, add 5-7mL of 1mol / L Na 2 CO 3 The pH of the solution was adjusted to about 8, and the two phases were separated. The organic phase was washed twice with 10 mL of distilled water and evaporated to dryness. Add 30 mL of dimethylformamide, 0.73 g (10 mmol) of diethylamine and 1.66 g (12 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com