Synthesis method of zolpidem tartrate impurities
A technology for zolpidem tartrate and zolpidem tartrate tablets is applied in the field of synthesizing zolpidem tartrate impurities, and can solve the problems of differences in the type and quantity of impurities, uneven quality and inconsistency of zolpidem tartrate tablets, and narrow the quality gap. , the effect of improving international competitiveness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Zolpidem (3.1 g, 10 mmoles) was suspended in 100 ml of dry THF solution, cooled to -78°C, and sodium hexamethyldisilazide was added dropwise to the THF solution (11.0 ml, 11.0 mmoles, 1.0M in THF), The dropwise addition was completed in 10 minutes. Stirring was continued for 2 hours, tert-butyl α-bromoacetate (1.61ml, 10.0mmoles) was added, the mixture was naturally raised to 25°C, and stirred overnight. On the next day, it was quenched by adding a saturated ammonium chloride solution dropwise, concentrated, and subjected to column chromatography to obtain impurity I and impurity II in zolpidem tartrate tablets respectively.
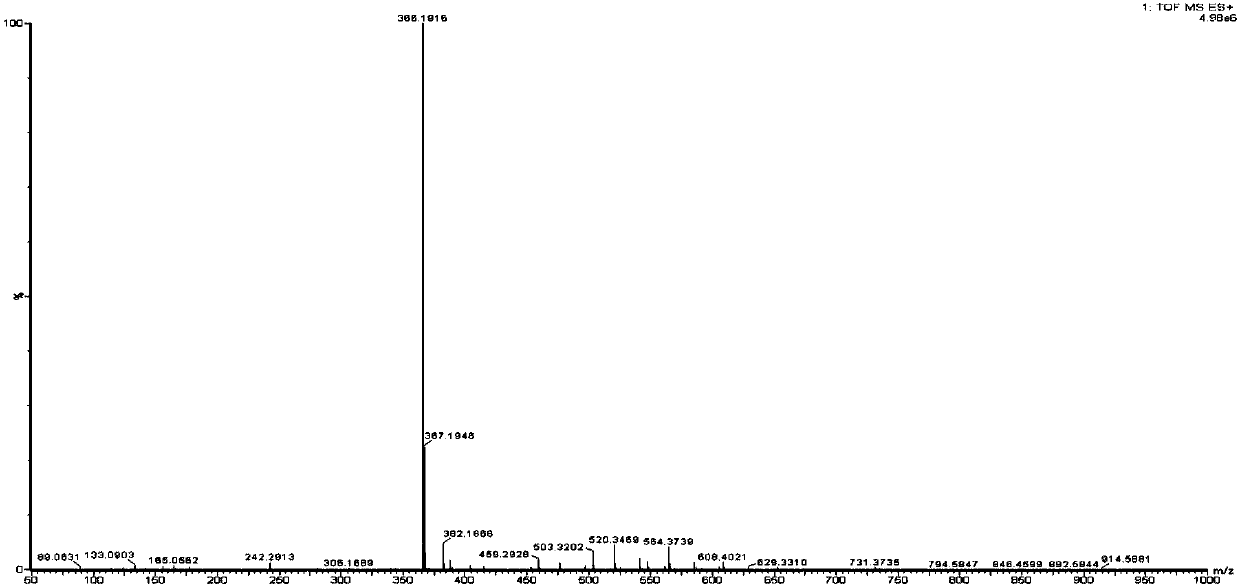
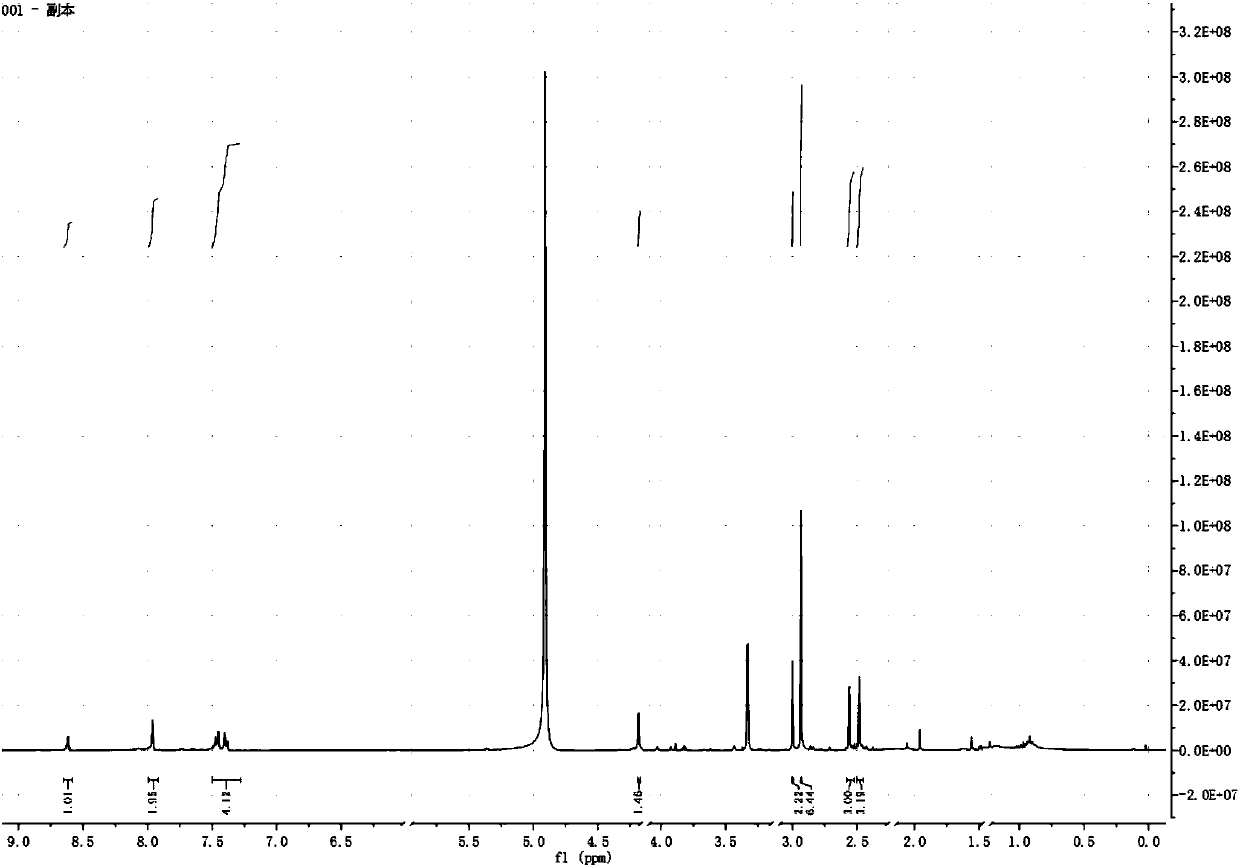
[0020] Impurity Ⅰ, m / z[M+H]+366.1916; 1H nmr(CD3OH,400MHz):δ2.47(s,3H,Ar-CH3),2.56(s,3H,Ar-CH3),2.93(s, 6H,N-(CH3)2),3.00(s,2H,CH2COOH),4.19(s,1H,Ar-CH),7.39(d,2H,Ar-H),7.45(d,2H,Ar-H ),7.96(s,2H,Ar-H),8.62(s,1H,Ar-H);
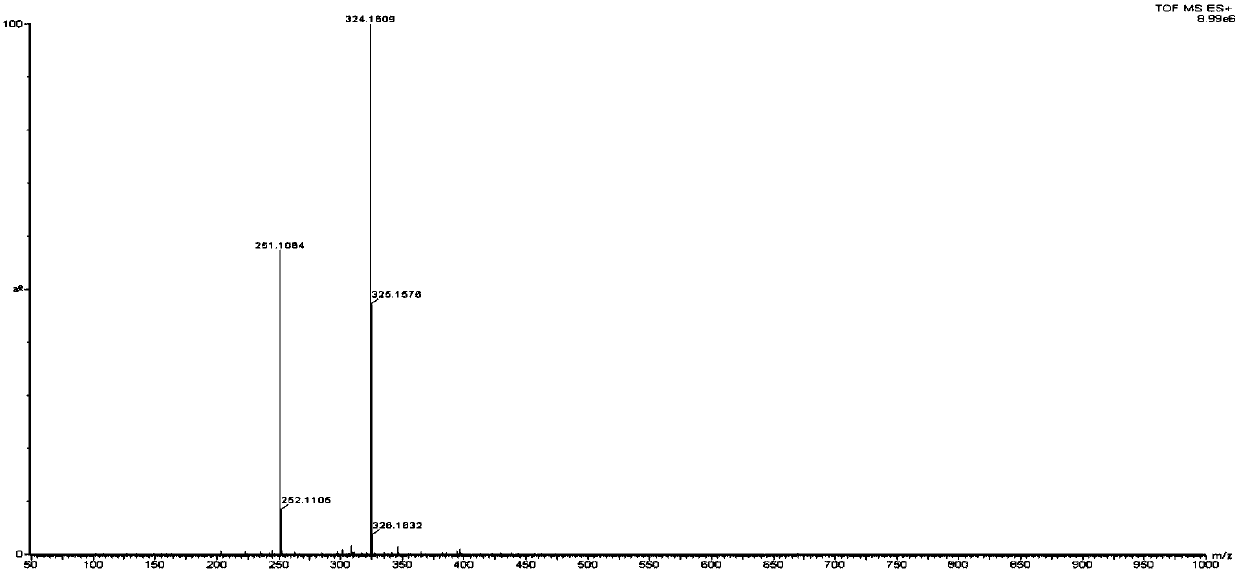
[0021] Impurity Ⅱ, m / z[M+H]+324.1609; 1H nmr(CDCl3,400MHz):δ2.24(s,3H,Ar-CH3),2.43(s,3H,Ar-CH3),2.69(s, 3H,N-CH3),2.87(s,3H,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com