Preparation method of near infrared fluorescence imaging micromolecule anticancer nano-drug
A nano-drug and fluorescence imaging technology, applied in the field of biomedicine, can solve the problems of unclear mechanism of action, complex nano-system, unclear metabolism, etc., and achieve the effect of improving photostability, simple preparation process, and eliminating clinical safety problems.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] The preparation method of ursolic acid nano micelles
[0046] Accurately weigh 0.00456g of UA powder, dissolve in 1ml of methanol, ultrasonically dissolve, and configure a 10 mM solution; take different volumes of methanol solution, and add dropwise to the solution containing 2 mL of secondary water (double distilled water) during the stirring process. ) (Note: Stir at high speed during the dropping process, and the dropping time is 30s), at this time, the concentration of UA in the solution is 31.25 μM-1000 μM, and then stir for 5 min to obtain UA nanomicelles;
[0047] The average particle size and PID of UA nanomicelles with different concentrations prepared in this example are shown in Table 1.
[0048] Table 1
[0049]
Embodiment 2
[0051] Accurately weigh 0.00853g of PTX powder, dissolve it in 1ml of methanol, and ultrasonically dissolve it to form a 10mM solution; take different volumes of methanol solution, and add it dropwise to 2ml of secondary water during the stirring process (note: the dropping process Medium-high speed stirring, dropping time is 30s), at this time, the concentration of PTX in the solution is 31.25μM-1000μM, and then stirring for 5min to obtain PTX nanomicelle;
[0052] The average particle size and PID of PTX nanomicelles with different concentrations prepared in this example are shown in Table 2.
[0053] Table 2
[0054]
Embodiment 3
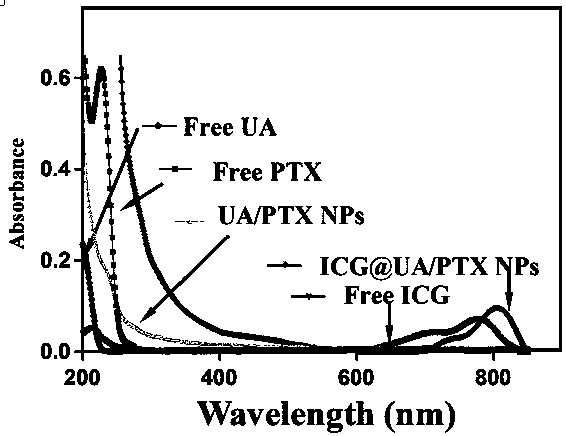
[0056] Accurately weigh 0.00456g of UA powder and 0.00853g of PTX powder, dissolve them in 1ml of methanol, configure them into a 10mM solution, and ultrasonically dissolve them; take different volumes of ursolic acid in methanol and different volumes of paclitaxel in methanol, mix them, and stir During the process, add dropwise to secondary water containing 2ml (note: stir at high speed during the dropping process, and the dropping time is 30s), and then stir for 5min to obtain UA / PTX nanomicelles with different molecular molar ratios;
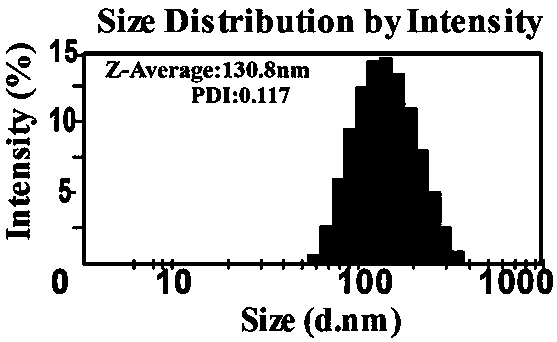
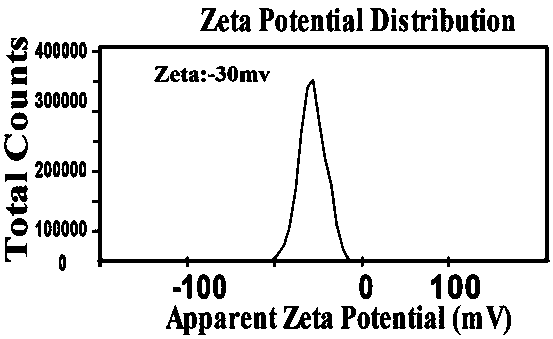
[0057] Table 3 shows the average particle size, PID and potential of the UA / PTX nanomicelles formed by different molecular molar ratios prepared in this example.
[0058] table 3
[0059]
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com