Tacrine-heteroauxin heterozygous compound as well as preparation method and application thereof
An indole acetic acid and compound technology, which is applied in the directions of organic chemistry, drug combination, nervous system diseases, etc., can solve the problems of large liver toxicity and side effects, and achieves the effect of simple preparation method and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Embodiment 1: the preparation of formula I compound
[0025]
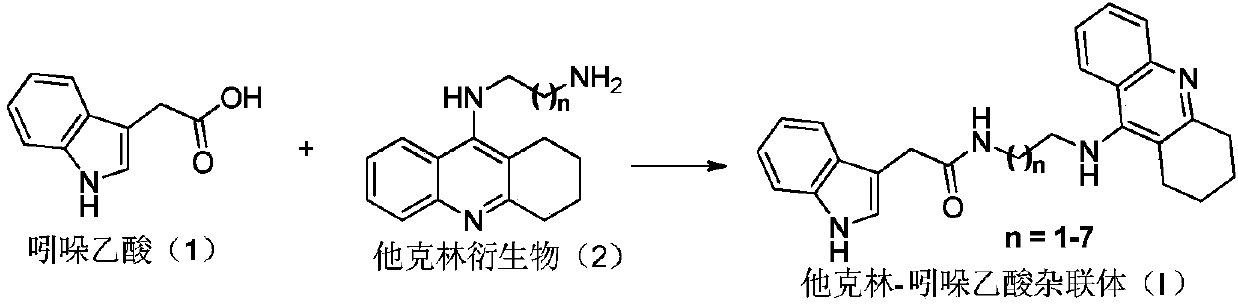
[0026] Dissolve 175mg indoleacetic acid (1, 1 equivalent) in 5ml dichloromethane, add 103mg N,N-diisopropylethylamine and 304mg 2-(7-azobenzotriazole)-tetramethyluronium hexafluoro Phosphate ester, stirred at room temperature for 3 h, and TLC detected that the reaction was complete. Add the corresponding tacrine derivative (2, 1 equivalent) dissolved in dichloromethane to the above reaction solution, stir at room temperature for three hours, TLC detects that the reaction is complete, concentrate the reaction solution under reduced pressure, and separate and purify by silica gel chromatography to obtain the corresponding A compound of formula I, wherein n=1-7. The specific compounds are as follows:
[0027] Compound of formula I-1
[0028] White solid (172mg, 43.3%), melting point 202.1-203.5°C. 1 H NMR (600MHz, MeOD) δ8.32(d, J=8.4Hz, 1H), 7.82(t, J=8.4Hz 1H), 7.70(d, J=8.4Hz, 1H), 7.51(t, J= 7.8Hz...
Embodiment 2
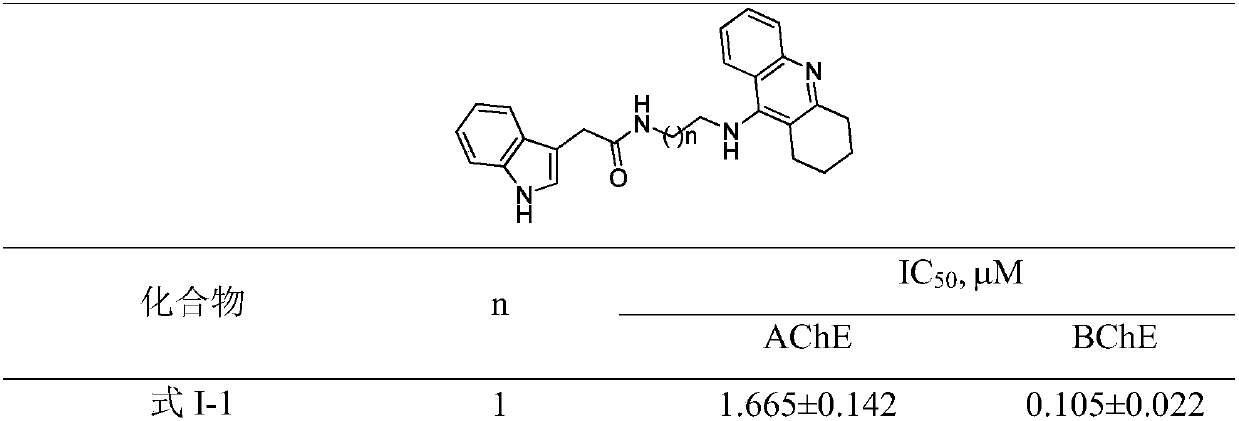
[0041] Example 2: Cholinesterase inhibitory activity
[0042] Reagents: acetylcholinesterase (AChE) or butyrylcholinesterase (BChE), thioacetylcholine iodide (ATC) or thiobutyrylcholine iodide (BUC) as substrate, and 5,5-Dithiobis(2-nitrobenzoic acid) (DTNB) was purchased from Sigma. The AChE and BChE inhibitory activities were measured with reference to the method reported by Ellan et al. (Ellman, G.L. et al. Biochem. Pharmacol. 1961, 7, 88.). Add 40 μL of phosphate buffer (pH=8.0) to each well of a 96-well plate, and then add 10 μL of 0.39, 0.78, 1.56, 3.125, 6.25, 12.5, 25, 50 and 100 M solutions of Formula I or blank control into the corresponding air , followed by adding 10 μL of AChE and incubating on a shaker at 37°C for 5 min. Add 20 μL of DTNB solution and incubate on a shaker at 37°C for 5 minutes, then add 10 μL of substrate ATC or BUC and incubate on a shaker at 37°C for 3 minutes, measure the absorbance at 412 nm with a microplate reader, and calculate the effec...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com