Recombinant adenovirus expressing anticancer peptide CB1a as well as construction method and application thereof
A technology of recombinant adenovirus and anticancer peptide, applied in the application field of medicine, can solve the problems of unstable structure, easy degradation of polypeptide medicine, high cost of chemical synthesis, simple construction method, inhibition of migration and growth, and repeatability. strong effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] The features and advantages of the present invention can be further understood through the following detailed description in conjunction with the accompanying drawings. The examples provided are only illustrative of the method of the present invention and do not limit the rest of the present disclosure in any way. [Example 1] Obtaining the target gene CB1a
[0051] 1. Primer design
[0052] Look up the amino acid sequence of CB1a on NCBI, and design and optimize the gene sequence using human-preferred codons. The optimized sequence is as follows:
[0053] ATGAAGTGGAAGGTGTTCAAGAAGATCGAGAAGAAGTGGAAGGTGTTCAAGAAGATCGAGAAGGCCGGCCCCAAGTGGAAGGTGTTCAAGAAGATCGAGAAGTGA (SEQ ID NO: 1).
[0054] Amino acid sequence of CB1a (MMDB ID: 144618):
[0055] KWKVFKKIEK KWKVFKKIEKAGPKWKVFKKIEK (SEQ ID NO: 1).
[0056] According to the above gene sequence, Primer Premier 5 was used to design overlapping PCR primers. In the second amplification primer, an EcoR I restriction site was added...
Embodiment 2
[0068] [Example 2] Preparation of recombinant adenovirus carrying CB1a gene
[0069] 1. Construction of recombinant adenovirus gene vector
[0070] 1.1 Construction and identification of pMD-20T-CB1a vector
[0071] CB1a and pMD-20T vector connection system: Solution I 5 μL, pMD-20T vector 0.5 μL, overlapping CB1a recovery product 2.6 μL, ddH 2 O 1.9 μL.
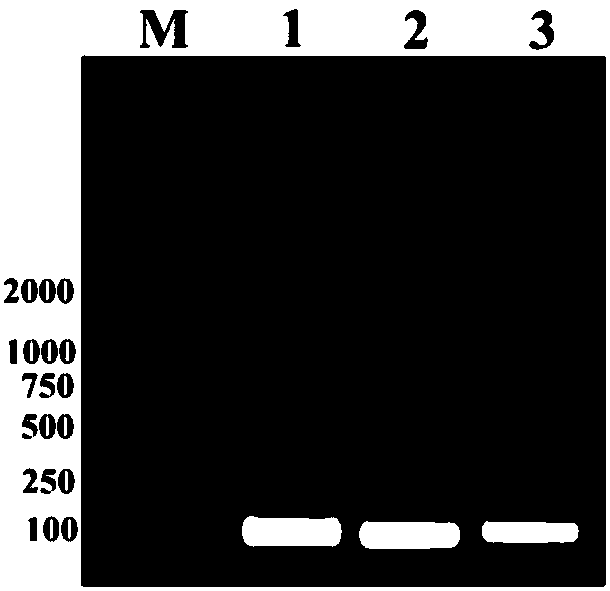
[0072] The ligated system was placed in a thermostat at 16°C overnight. EcoR I, Xba I double enzyme digestion identification, the reaction product is electrophoresed with 1% agarose gel (such as figure 2 , about 100bp band is the CB1a gene). Sequencing identification was performed by Wuhan Sequencing Department of Sangon Bioengineering (Shanghai) Co., Ltd.
[0073] 1.2 Construction and identification of pAdTrack-CB1a shuttle vector
[0074] The correct pMD-20T-CB1a and pAdTrack-CMV were digested with EcoR I and Xba I at the same time, and CB1a was recovered by agarose gel electrophoresis, and the linearized pAdTrack-C...
Embodiment 3
[0119] [Example 3] Detection of inhibition of cancer cells
[0120] 1. MTT detection of recombinant adenovirus infected cancer cells
[0121] ① Place 5000 cancer cells / well into a 96-well plate at 37°C, 5% CO 2 Cultivate for 12h.
[0122] ② Add viruses with different multiplicity of infection, and set 5 replicates for each multiplicity of infection.
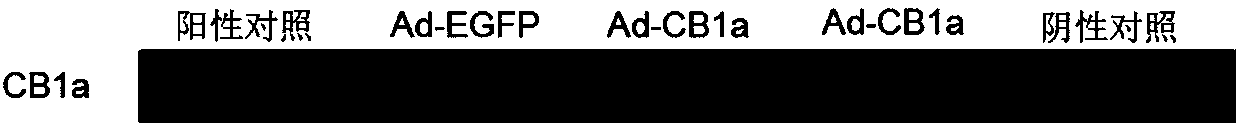
[0123] ③ Add 20 μL MTT (5 mg / mL) to culture for 48 hours, 72 hours, and 96 hours respectively, and culture for 4 hours; then remove the medium, add 200 μL DMSO to completely dissolve the purple crystals, measure the absorbance at 490 nm, and Record data to analyze cell viability. SCC9 cells (such as Figure 5 ), LN229 cells (such as Figure 6 ), U87 cells (such as Figure 7 ), A549 cells (such as Figure 8 ) experiment, the results showed that the inhibitory effect after 72h and 48h after adding the recombinant adenovirus was significantly higher than that of the control group, and the different MOIs were slightly differen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com