Recycling of ionic liquid in key reaction for cantharidin preparation
An ionic liquid, key reaction technology, applied in organic compound/hydride/coordination complex catalysts, organic chemistry, physical/chemical process catalysts, etc., can solve the problems of difficult target product separation, high price, etc. The effect of low cost and lower production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Cantharidin intermediate synthesis:
[0028] 100g 2,5-dihydrothiophene-3,4-dicarboxylic anhydride, 600ml furan, 400ml ionic liquid 1-butyl-3-methylimidazolium tetrafluoroborate, catalytic amount of boron trifluoride ether , added to the reactor, heated to 50° C. and stirred for 18 hours, then cooled to room temperature to obtain a reaction system.
[0029] Ionic liquid recovery:
[0030] The reaction system was extracted three times with acetonitrile, the amount of acetonitrile used was 600 ml, and the temperature of the extraction system was kept at 40°C. After mixing the extracts, they were distilled under reduced pressure to obtain the recovered ionic liquid.
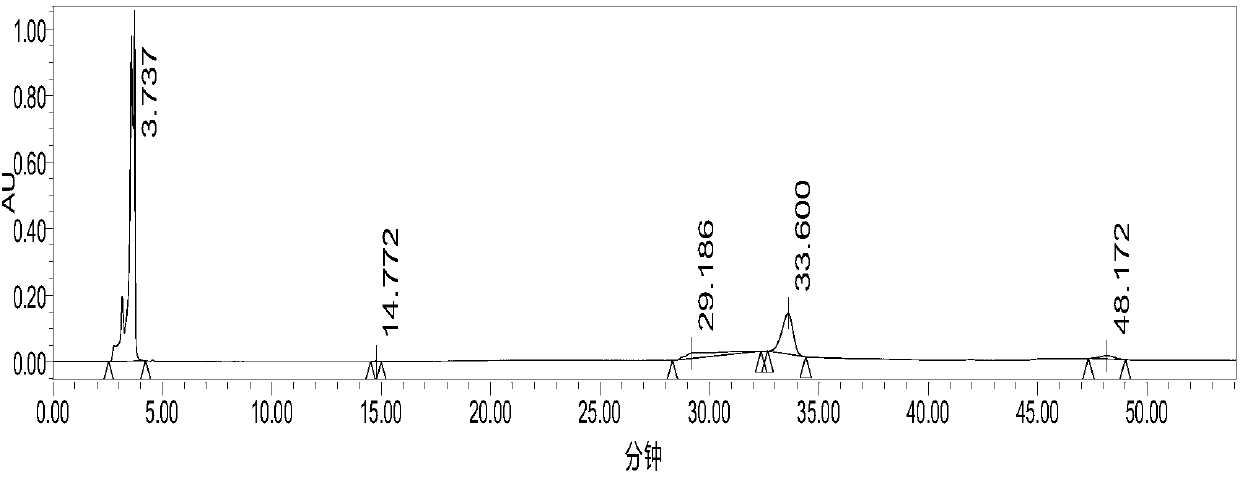
[0031] Ionic liquid 1-butyl-3-methylimidazolium tetrafluoroborate recovery rate is 80%; HPLC figure ( figure 1 )
[0032] The yield of cantharidin intermediate was 94%.
[0033] It can be seen from the data that the cantharidin intermediate product in the key step of cantharidin is effectively separated, ...
Embodiment 2
[0035] Cantharidin intermediate synthesis:
[0036] 50g of 2,5-dihydrothiophene-3,4-dicarboxylic anhydride, 175ml of furan, 100ml of reclaimed ionic liquid (1-butyl-3-methylimidazolium tetrafluoroborate recovered in Example 1), A catalytic amount of boron trifluoride diethyl ether was added into the reactor, stirred and reacted at room temperature for 25 hours, and cooled to room temperature to obtain a reaction system.
[0037] Ionic liquid recovery:
[0038] The reaction system was extracted three times with acetonitrile, the total amount of acetonitrile was 875 ml, and the temperature of the extraction system was kept at 25°C. After mixing the extracts, they were distilled under reduced pressure to obtain the secondary recovery ionic liquid.
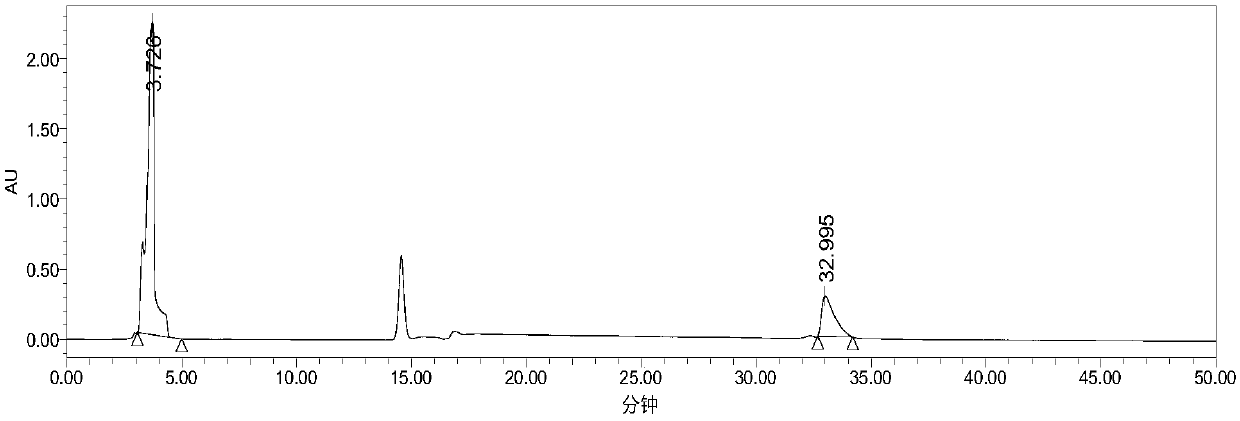
[0039] Secondary reclaiming ionic liquid after this application, its ionic liquid 1-butyl-3-methylimidazolium tetrafluoroborate rate of recovery is 70%; HPLC figure ( figure 2 ).
[0040] The yield of cantharidin intermediate is ...
Embodiment 3
[0043] Cantharidin intermediate synthesis:
[0044] 20g 2,5-dihydrothiophene-3,4-dicarboxylic anhydride, 120ml furan, 60ml ionic liquid 1-butyl-3-methylimidazole bis(trifluoromethylsulfonimide), catalytic amount of Boron trifluoride diethyl ether was added into the reactor, heated to 35°C and stirred for 20 hours, then cooled to room temperature to obtain a reaction system.
[0045] Ionic liquid recovery:
[0046] The reaction system was extracted 3 times with acetonitrile, the total amount of acetonitrile was 60ml, and the temperature of the extraction system was kept at 30°C. After mixing the extracts, they were distilled under reduced pressure to obtain the recovered ionic liquid.
[0047] The recovery rate of the ionic liquid 1-butyl-3-methylimidazole bis(trifluoromethylsulfonimide) was 91%; the yield rate of the cantharidin intermediate was 92%.
[0048] The same product can be obtained by repeating the reaction of the ionic liquid according to the above steps, and a c...
PUM
| Property | Measurement | Unit |
|---|---|---|
| recovery rate | aaaaa | aaaaa |
| recovery rate | aaaaa | aaaaa |
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com