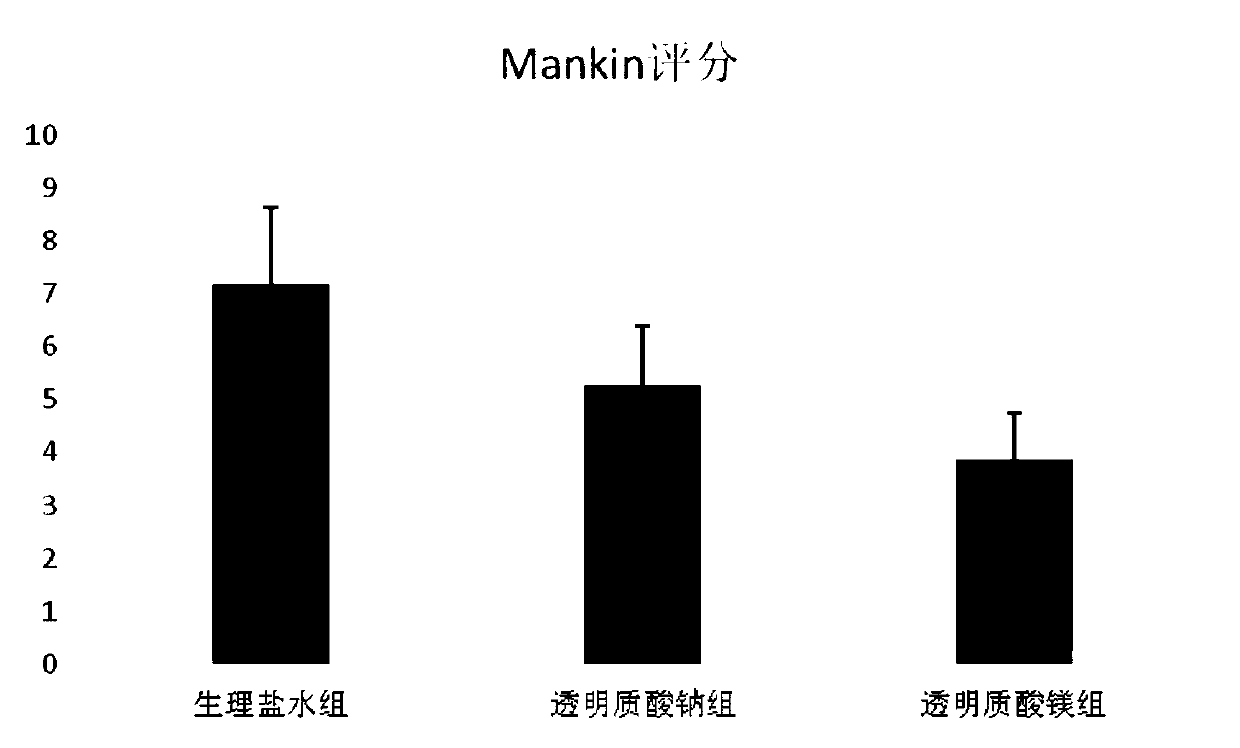
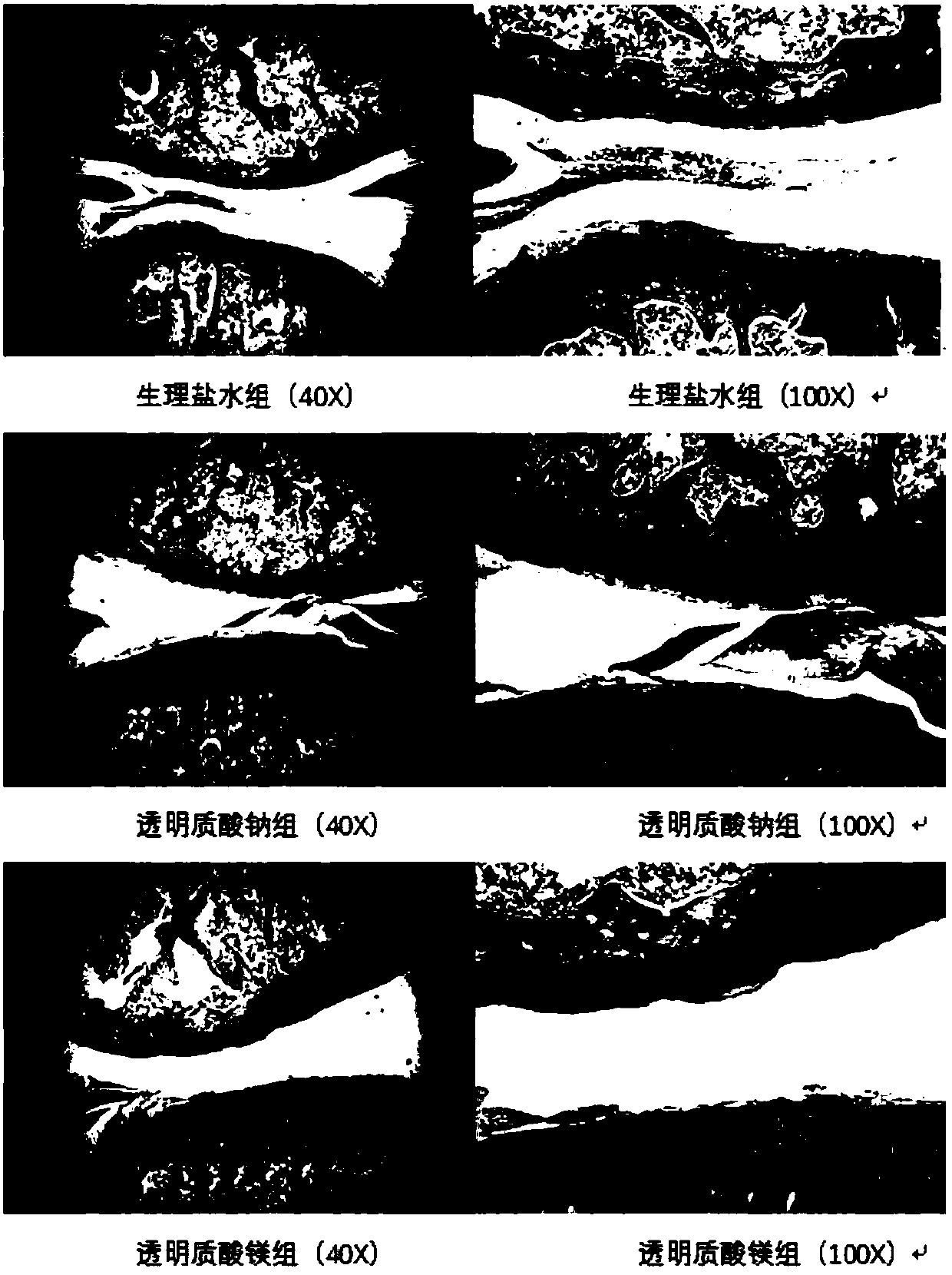
Magnesium hyaluronate for treating osteoarthritis and preparation method and application thereof
A technology for magnesium hyaluronate and osteoarthritis, which is applied to medical preparations containing active ingredients, anti-inflammatory agents, and pharmaceutical formulas, and can solve problems such as inaccurate efficacy of intra-articular injection of sodium hyaluronate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1 Preparation of Magnesium Hyaluronate
[0026] 1. Take 300mg of magnesium acetate and dissolve it completely in 5ml of distilled water.
[0027] 2. Put 100mL of ethanol into the magnesium acetate solution, stir evenly, and heat to reach 40°C.
[0028] 3. While stirring, add 500 mg of sodium hyaluronate and react at a temperature of 40°C for 60 minutes.
[0029] 4. After the reaction is over, let it stand for 15 minutes, pour off the supernatant, and centrifuge to obtain a precipitate.
[0030]5. Take the precipitate, add 90% (v / v) ethanol with 5 times the mass of the precipitate, stir for 10 minutes, let stand for 15 minutes, and centrifuge to obtain the precipitate.
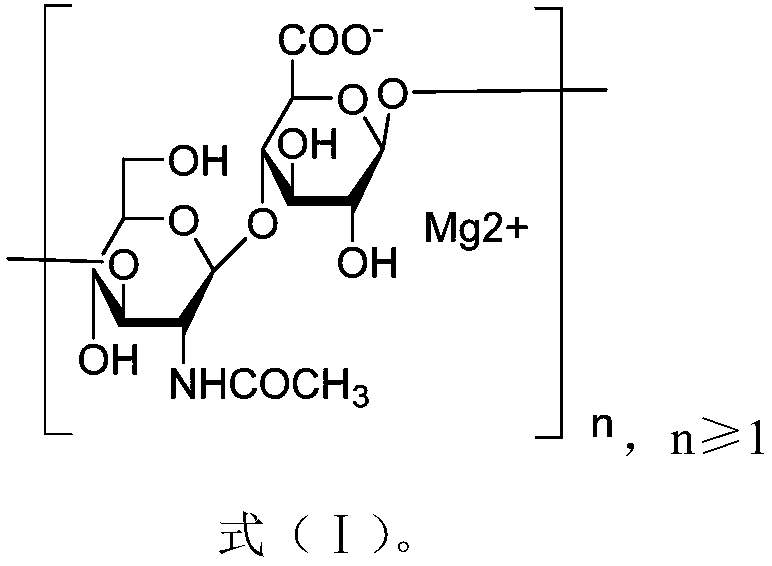
[0031] 6. Step 5 was repeated 5 times, and the obtained precipitate was vacuum-dried at 60° C. to obtain 450 mg of magnesium hyaluronate, and the yield (%) of magnesium hyaluronate = 450 / 500 = 90%. The structure is as formula (Ⅰ):
[0032]
Embodiment 2
[0033] Example 2 In vitro cytotoxicity evaluation of magnesium hyaluronate for chondrocytes
[0034] 1 Experimental materials and methods
[0035] 1.1 Drugs and reagents
[0036] Magnesium Hyaluronate, Sodium Hyaluronate, Normal Saline
[0037] Cell Counting Kit (CCK-8 Kit)
[0038] 1.2 cells
[0039] mouse chondrocytes
[0040] 1.3 Experimental method
[0041] 1.3.1 Take mouse chondrocytes growing in the logarithmic phase, inoculate 5000 cells / well in 96-well culture plate, each well volume is about 100 μl, in 5% CO 2 , 37°C incubator for about 24h until the cells grow to a monolayer. Then, discard the supernatant in the well, wash with phosphate buffered solution (PBS) for 1-2 times, add complete medium (DMEM / F12+10% fetal bovine serum) and a series of drug solutions (100 μl ), were divided into 5 groups, and each group had 3 replicate wells. The cell plate was further incubated in the incubator for 48 h. Grouped as follows:
[0042] 1) 5ng / ml magnesium hyaluronate...
Embodiment 3
[0052] Example 3 Preparation of magnesium hyaluronate intra-articular injection
[0053] At room temperature, dissolve the magnesium hyaluronate synthesized according to Example 1 with physiological saline, and dilute to a corresponding concentration of 1%, filter through a 0.22 μm filter membrane, potting, sterilizing, inspecting and packaging.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com