Pharmaceutical composition for preventing and treating ovarian cancer
A composition and a technology for ovarian cancer, applied in the field of medicine, can solve the problems of no literature report on terazosin hydrochloride ovarian cancer, no literature report on terazosin hydrochloride and the like, and achieve significant social and economic significance, tumor-inhibiting activity. Significant, less toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Embodiment 1: take by weighing cytarabine hydrochloride 125g, terazosin hydrochloride 50g, starch 200g, microcrystalline cellulose 25g, talcum powder 3g, cytarabine hydrochloride, terazosin hydrochloride cross 120 mesh sieves, starch 1. The microcrystalline cellulose is passed through a 100-mesh sieve, weighed according to the prescribed amount, mixed evenly, granulated, dried, granulated, added with prescribed amount of talcum powder, mixed evenly, and filled to obtain capsules.
Embodiment 2
[0016] Embodiment 2: take by weighing cytarabine hydrochloride 200g, terazosin hydrochloride 50g, starch 200g, microcrystalline cellulose 25g, talcum powder 3g, cytarabine hydrochloride, terazosin hydrochloride cross 120 mesh sieves, starch 1. The microcrystalline cellulose is passed through a 100-mesh sieve, weighed according to the prescribed amount, mixed evenly, granulated, dried, granulated, added with prescribed amount of talcum powder, mixed evenly, and filled to obtain capsules.
Embodiment 3
[0017] Embodiment 3: The combination of terazosin hydrochloride and cytarabine hydrochloride
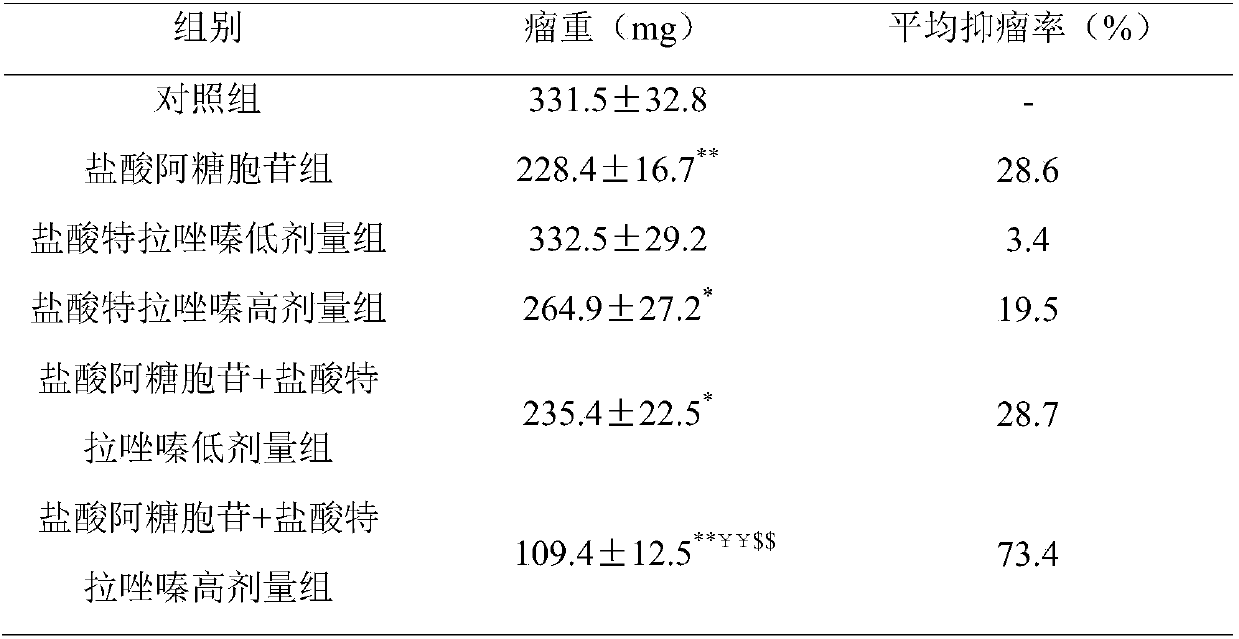
[0018] 40 SPF grade BALB / c-nu mice, 6 weeks old, weighing 18g-20g. Absorb and discard the medium in the bottle of SKOV3 ovarian cancer cells in the logarithmic growth phase with a confluence of 80% to 90%, wash the cells 2-3 times with phosphate buffer saline, digest with trypsin (0.25%), collect the cells, and again Wash with phosphate buffer, blow and beat to mix, and count; then adjust the concentration with serum-free RPMI-1640, and inoculate 1.0×10 cells subcutaneously on the back of each nude mouse 7 / 0.2mL cell suspension was injected subcutaneously on the back of nude mice. From 7 days after inoculation, small nodules were observed in the subcutaneous area of the tumor cell inoculation site, with a size of about 5mm×5mm; on the 10th day, the size of the tumor was about 7mm×7mm.
[0019] Thirty-five nude mice with tumors were taken and divided into 7 groups by random and b...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com