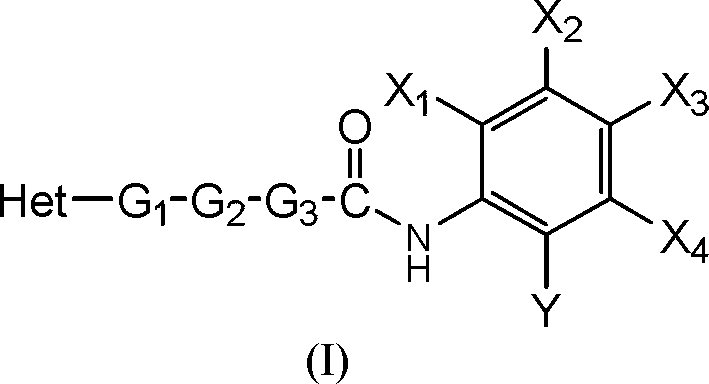
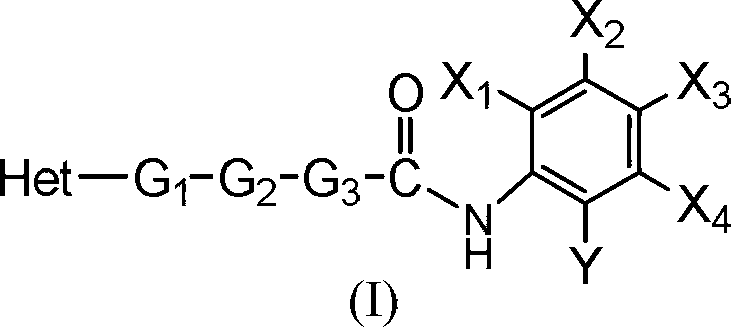
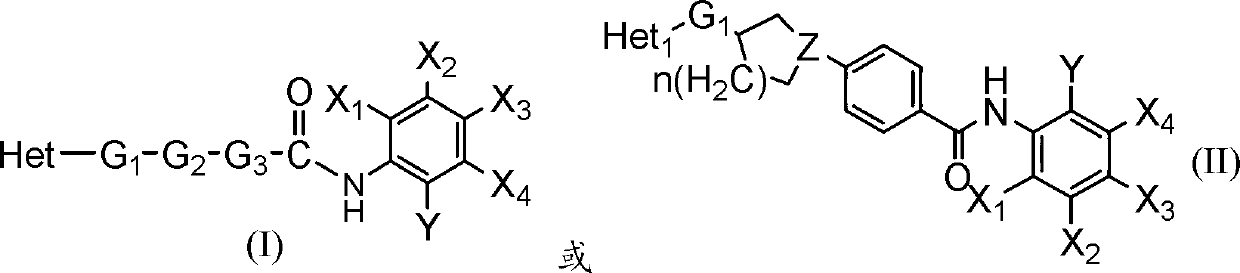Benzamide derivative resisting proliferative activity and pharmaceutical preparation thereof
A benzamide and pharmacy technology, applied in the field of new benzamide derivatives and preparation thereof, can solve the problems of unsuitability for large-scale preparation, large side effects and high production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0205] Example 1. Synthesis of N-(2-amino-5-fluorophenyl)-4((4-(pyridin-3-yl)pyrimidine-2-amino)methyl)benzamide (compound 1)
[0206] Step 1. Synthesis of 3,3-Dimethylamino-1-pyridin-3-yl-propenone
[0207] Dissolve 2g of 3-acetylpyridine in 5ml of anhydrous N,N-dimethylformamide, then add 5ml of N,N-dimethylformamide diacetal to the solution, and heat to 110°C for reaction 3 Hours, thin-layer chromatography showed that the reaction was complete, N,N-dimethylformamide was recovered under reduced pressure, the residual liquid was placed in the refrigerator overnight, a yellow solid was precipitated, filtered by suction, washed with ethyl acetate / petroleum ether 1:1, to obtain Yellow solid 2.05g.
[0208] The melting point of the product is m.p.82-83°C;
[0209] Step 2. Synthesis of 4-guanidinomethyl-benzoic acid
[0210] Dissolve 2g of methylisothiourea sulfate in 10ml of 1mol / L sodium hydroxide aqueous solution, slowly add 2.15g of 4-aminomethylbenzoic acid dropwise under ...
Embodiment 2
[0260] Example 2. Synthesis of N-(2-amino-5-fluorophenyl)-4-((4-methoxyphenylamino)methyl)benzamide (compound 5)
[0261] Step 1. Synthesis of methyl p-aldehyde benzoate
[0262] Dissolve 2.0 g of p-aldehyde benzoic acid in 20 ml of methanol, slowly add 1.81 ml of thionyl chloride dropwise under ice bath, after the addition is completed, after heating and refluxing for 3 hours, thin layer chromatography shows that the reaction is complete, and the solvent is recovered under reduced pressure. Cool and filter to obtain 2.13 g of light yellow solid.
[0263] The melting point of the product is m.p.59-61°C;
[0264] Step 2. Synthesis of methyl 4-((4-methoxyphenylamino)methyl)benzoate hydrochloride
[0265] Dissolve 1.59g of p-aminoanisole and 2.13g of the product of step 1 in 15ml of methanol, and add 3.07g of NaBH in batches under ice-cooling 4 , and dropwise added glacial acetic acid to keep the pH of the reaction solution at 5-6, thin-layer chromatography showed that the rea...
Embodiment 3
[0304] Example 3. Synthesis of 4-(((phenylethyl)(2-hydroxyethyl)amino)methyl)-N-(2-amino-5-fluorophenyl)benzamide (compound 8)
[0305] Step 1. Synthesis of methyl 4-((phenethylamino)methyl)benzoate hydrochloride
[0306] Dissolve 1.43ml of phenethylamine and 1.46g of methyl p-aldehyde benzoate in 10ml of methanol, add 2.5g of sodium borohydride in batches under ice bath, and add dropwise glacial acetic acid to keep the pH of the reaction solution at 5-6, thin layer Chromatography showed that the reaction was complete, and the solvent was removed under reduced pressure. The residue was distributed in ethyl acetate / water and made acidic with 2N hydrochloric acid. A large amount of white solid was precipitated, which was filtered and dried to obtain 2.06 g of white solid.
[0307] Step 2. Synthesis of methyl 4-(((2-(tert-butyldimethylsilyloxy)ethyl)(phenethyl)amino)methyl)benzoate
[0308] Take a 100ml dry three-necked bottle, under the protection of dry nitrogen, dissolve 2.06...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com