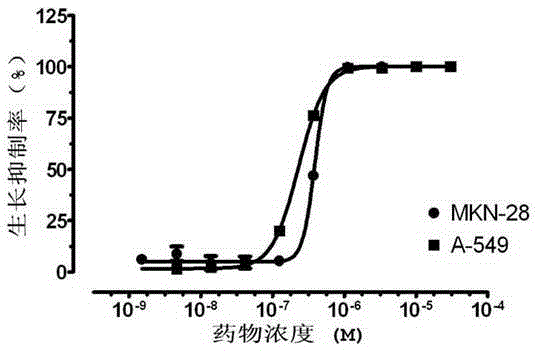
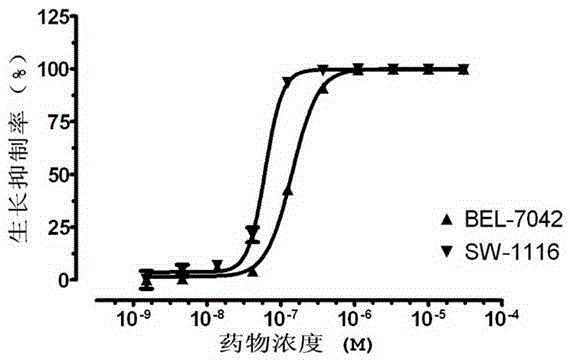
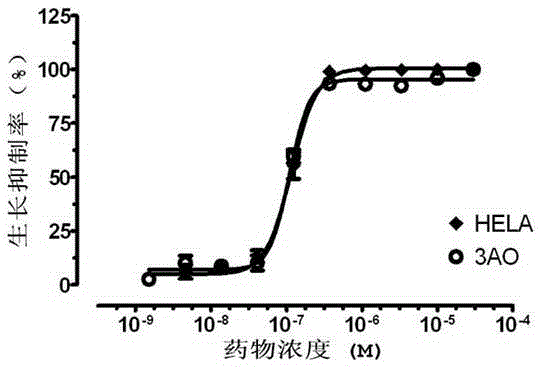
Bismuth (Ⅲ) complex of substituted pyrrolidinylcarbamate and its preparation method and application in the preparation of antitumor drugs
A technology of bismuth pyrrolidine amino acid and complexes, which is applied in the field of anti-tumor drugs, can solve the problems of few anti-tumor cell lines, single structure, low clinical application value, etc., and achieves significant anti-tumor activity, wide application prospect, good The effect of antitumor activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment one: (2R)-2-Methylpyrrolidine bismuth dicarbamate and its preparation
[0032] Add 0.426g (5mmol) (2R)-2-methylpyrrolidine, 0.25g (6mmol) sodium hydroxide, 15mL anhydrous methanol into a round bottom flask, stir and dissolve with a magnetic force, put in a cold water bath at 0-5°C, add dropwise 0.76g (10mmol) CS 2 , Magnetic stirring reaction 5h.
[0033] 0.53g (1.7mmol) BiCl 3 Dissolve in 15 mL of anhydrous methanol, drop into the above reaction solution, stir at room temperature for 3 h, filter with suction, wash with anhydrous methanol, and dry in vacuo. The solid was recrystallized from dichloromethane and ethanol to obtain 0.82 g of a yellow powder solid with a yield of 71.3%. Melting point: 224-226°C, specific rotation =-205 ° (HCl 3 ), number N062.
[0034] 1 HNMR (400MHz, CDCl 3 ),δ:1.362~1.378(9H,d,-CH 3 ,J=6.4Hz),1.692~1.731(3H,m,Cy-CH,J=5.2Hz),2.035~2.071(3H,m,Cy-CH,J=4.0-4.8Hz),2.088~2.161(6H ,m,Cy-CH 2 -,J=4.0-6.8Hz),3.890~3.952(6H...
Embodiment 2
[0036] Embodiment two: (S)-2-Carboxypyrrolidinyl bismuth dicarbamate and its preparation
[0037] Add 0.576g (5mmol) L-proline, 0.759g (7.5mmol) triethylamine, and 15mL anhydrous methanol into a 50mL round bottom flask, stir and dissolve with magnetic force, and add 0.76g ( 10mmol) CS 2 , magnetically stirred for 1 h, stirred at room temperature for 3 h.
[0038] 0.53g (1.7mmol) BiCl 3 Dissolve in 15mL of anhydrous methanol, drop into the above reaction solution, and stir at room temperature for 4h. Use glacial acetic acid to adjust the pH value to 5-6, and a yellow precipitate precipitates, which is suction filtered, washed with anhydrous methanol, and dried. The solid was recrystallized from methanol to obtain 1.05 g of a yellow solid. Yield 80.8%. Melting point: 186-188℃, specific rotation =-103 ° (DMSO).
[0039] 1 HNMR (400MHz, DMSO- d 6 ), δ: 1.971~2.014 (6H, m, Cy-CH 2 ,J=6.4~7.2Hz), 2.272~2.318(6H,m,Cy-CH 2 , J=6.4-7.6Hz), 3.818~3.848(6H,t,N-CH 2 , J=7...
Embodiment 3
[0041] Embodiment three: (S)-2-Ethoxycarbonyl pyrrolidinyl bismuth dicarbamate and its preparation
[0042] Add 0.898g (5mmol) L-proline ethyl ester hydrochloride, 0.44g (11mmol) sodium hydroxide, and 15mL anhydrous methanol into a 50mL round-bottomed flask. Add 0.76g (10mmol) CS 2 , magnetically stirred for 2h, stirred at room temperature for 4h.
[0043] 0.53g (1.7mmol) BiCl 3 Dissolve in 15 mL of anhydrous methanol, drop into the above reaction solution, stir at room temperature for 3 h, filter with suction, wash with anhydrous methanol, and dry in vacuo. The solid was recrystallized from acetonitrile to obtain 1.15 g of bright yellow crystals. Yield 79.9%. Melting point: 89-91℃, specific rotation =-393 ° (CHCl 3 ), number N048.
[0044] 1 HNMR (400MHz, CDCl 3 ),δ:1.261~1.297(9H,t,-CH 3 ,J=7.2Hz),2.069~2.091(3H,m,Cy-CH, J=4.4-6.8Hz),2.092~2.155(6H,m,Cy-CH 2 ,J=5.6-8.2Hz),2.314~2.400(3H,m,Cy-CH,J=6.4-8.0Hz),3.929~3.998(3H,m,N-CH,J=4.4-7.6Hz),4.061 ~4.108(3H,m...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com