Iron sucrose injection and preparation method thereof
A technology of iron sucrose and injection, which is applied in the directions of blood diseases, pharmaceutical formulations, extracellular fluid diseases, etc., can solve the problems of energy consumption, large discharge pollution, unstable product quality, low product yield and other problems in the production process, and achieves a high level of product quality. High yield, low impurity content and good quality stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
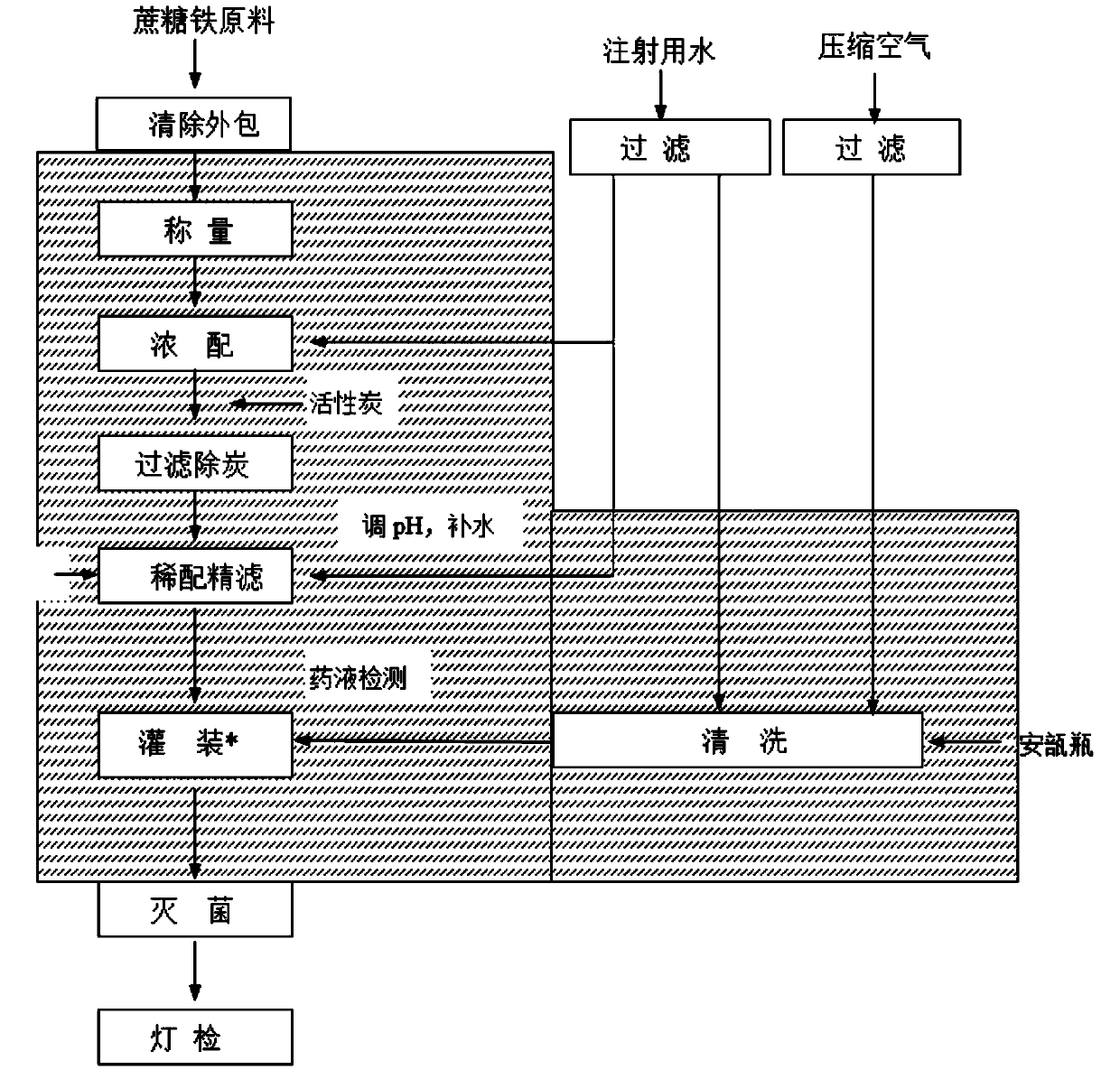
[0038] A kind of preparation method of iron sucrose injection of the present invention comprises:
[0039] figure 1 For the preparation flowchart of iron sucrose injection in a kind of embodiment of the present invention, please refer to figure 1 .
[0040] Iron sucrose injection is a small-volume injection, and there is no special requirement on the osmotic pressure of the preparation. However, in order to minimize the risk of medication due to inappropriate osmotic pressure, it should be as close as possible to the osmotic pressure of normal human blood (285-310mOsmol / kg). Therefore, the dosage of the isotonic regulator of the preparation is screened here.
[0041] Before the research on the prescription system of the preparation, the interaction between the main drug and the excipients should be investigated first. For injections, the pH of the system will not only affect the solubility of the main drug, but also affect the stability of the main drug. Ultimately affect...
Embodiment 1
[0055] This embodiment provides a preparation method of iron sucrose injection. In the present embodiment, the formulation prescription is as follows:
[0056] 1.5 g of iron sucrose raw material, an appropriate amount of 1M sodium hydroxide, and water as a solvent, so that the pH value of the entire dispersion system was adjusted to 11, and 5 ml of the first dispersion system was obtained.
[0057] No activated carbon was added to the first dispersion.
[0058] When the iron sucrose raw material and sodium hydroxide are mixed with water as the solvent, the temperature is maintained at 80°C when the diluted mixing tank is used for dilute mixing.
[0059] For injections, the pH of the system will not only affect the solubility of the main drug, but also affect the stability of the main drug. Ultimately affect the safety of medication. Therefore, the influence of pH on the stability of the main drug is very important. In this embodiment, the preparation sequence is:
[0060] K...
Embodiment 2
[0065] This embodiment provides a preparation method of iron sucrose injection. In the present embodiment, the formulation prescription is as follows:
[0066] 1.8 g of iron sucrose raw material, an appropriate amount of 1M sodium hydroxide, and water as a solvent, so that the pH value of the entire dispersion system was adjusted to 11.2, and 5 ml of the first dispersion system was obtained.
[0067] No activated carbon was added to the first dispersion.
[0068] When mixing the raw material of iron sucrose and sodium hydroxide with water as the solvent, the temperature is maintained at 85°C during the dilution in the dilution tank.
[0069] For injections, the pH of the system will not only affect the solubility of the main drug, but also affect the stability of the main drug. Ultimately affect the safety of medication. Therefore, the influence of pH on the stability of the main drug is very important. In this embodiment, the preparation sequence is:
[0070] Keep the te...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com