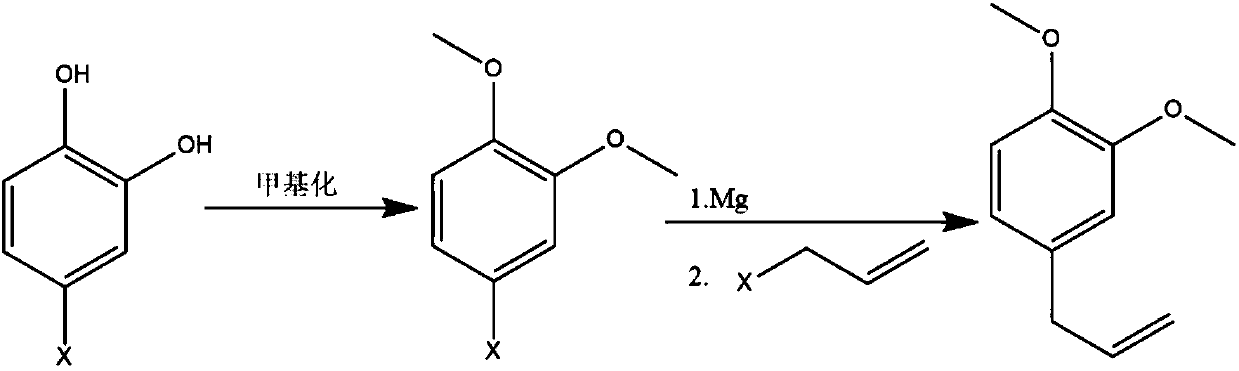
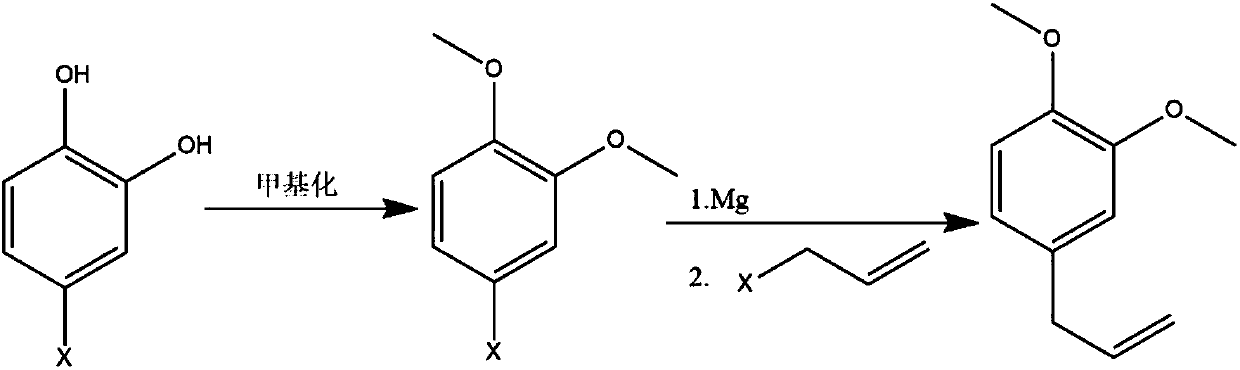
Method for synthesizing methyleugenol
A technology for synthesizing eugenol methyl ether, which is applied in chemical instruments and methods, preparation of ether, preparation of ether by ester reaction, etc., can solve problems such as high-purity separation difficulties, environmental hazards, and dimethyl sulfate is highly toxic, and achieves Simple purification, reduced environmental pollution, and less toxic effects on the human body
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Example 1. Dissolve 144.5g (1mol, 1eq) of 4-chloroquinone in 500ml toluene and put it into a reaction flask, then add 5.6g KOH, 5g K ion-type molecular sieve and 180g dimethyl carbonate, and open 1g TBAB Stir and heat to 70-80°C, react for 8-12 hours, TLC (silica gel plate chromatography separation, commonly known as climbing plate) to confirm the completion of the reaction, cool, filter, distill, add 10% hydrochloric acid to adjust the pH to 7, and then use n-hexane Extract, dry and distill to obtain 162g of 4-chloroquinone methyl ether with a purity of 95%.
[0020] The obtained 162g of 4-chloroquinone methyl ether was dissolved in 300ml of anhydrous ether for subsequent use. Put 24g of Mg pin into the reaction bottle, add 300ml of anhydrous ether and 2 iodine, start stirring, nitrogen protection, drop 20ml of 4-chloroquinone methyl ether, and then add 3~5ml of dibromoethane . After the reaction was initiated, the remaining anhydrous diethyl ether solution of 4-chlo...
Embodiment 2
[0022] Example 2. Dissolve 144.5g (1mol, 1eq) of 4-chloroquinone in 500ml of toluene and put it into a reaction flask, then add 10% aqueous solution containing 28g of KOH and 180g of dimethyl carbonate, 1g of TBAB is turned on, stirred and heated to reflux , reacted for 8 to 12 hours, after confirming the completion of the reaction, cooled, adjusted to PH=7 with 10% hydrochloric acid, then extracted with n-hexane, dried and distilled to obtain 138g of 4-chloroquinone methyl ether with a purity of 97%.
[0023] The obtained 138g of 4-chloroquinone methyl ether was dissolved in 300ml of anhydrous ether for subsequent use. Put 20g of Mg pin into the reaction bottle, add 300ml of anhydrous ether and 2 grains of iodine, start stirring, nitrogen protection, drop 20ml of 4-chloroquinone methyl ether, and then add 3~5ml of dibromoethane . After the reaction was initiated, the remaining anhydrous diethyl ether solution of 4-chloroquinone methyl ether was added dropwise. After the reac...
Embodiment 3
[0025] Example 3. Dissolve 2kg of 4-chloroquinone in 10L of toluene and put it into a reaction kettle, then add 553g of NaOH, 50g of K ion-type molecular sieve and 2.5Kg of dimethyl carbonate, and start stirring with 50g of TBAB and heat to 70-80 ℃, reacted for 8 to 12 hours, after confirming that the reaction was complete, cooled, filtered, and distilled, adjusted to PH=7 with 15% hydrochloric acid, then extracted with n-hexane, dried, and distilled to obtain 2.26Kg 4-chloroquinone methyl ether, 96% purity.
[0026] The obtained 2.26Kg of 4-chloroquinone methyl ether was dissolved in 5L of n-hexane for later use. Put 320g of Mg pin into the reaction bottle, add 5L of anhydrous ether and 2 grains of iodine, start stirring, nitrogen protection, drop 300ml of 4-chloroquinone methyl ether, and then add 10-20ml of dibromoethane . After the reaction was initiated, the remaining anhydrous diethyl ether solution of 4-chloroquinone methyl ether was added dropwise. After the reaction...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com