Preparation method for fluoromalonic acid diester
A technology of fluoromalonate diester and fluoroacetate ester is applied in the field of preparation of fluoromalonate diester, and can solve the problems of strong corrosion of equipment, high requirement of reaction equipment, environmental pollution and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
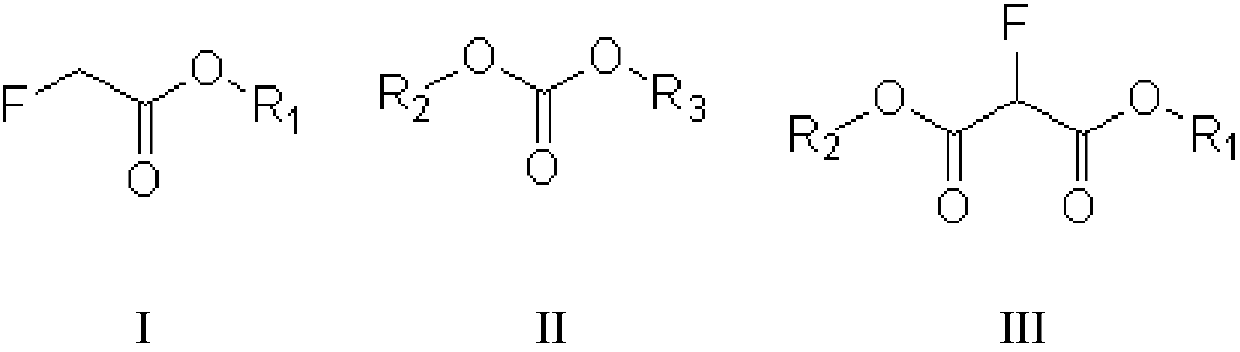
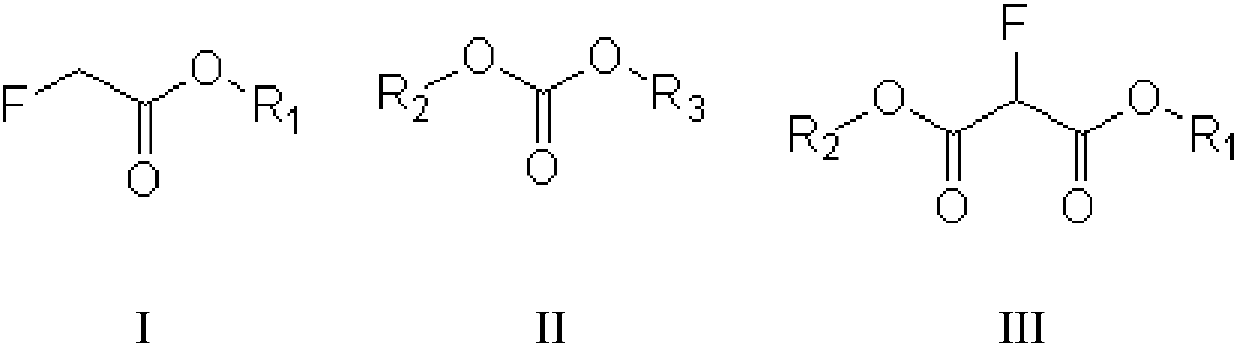
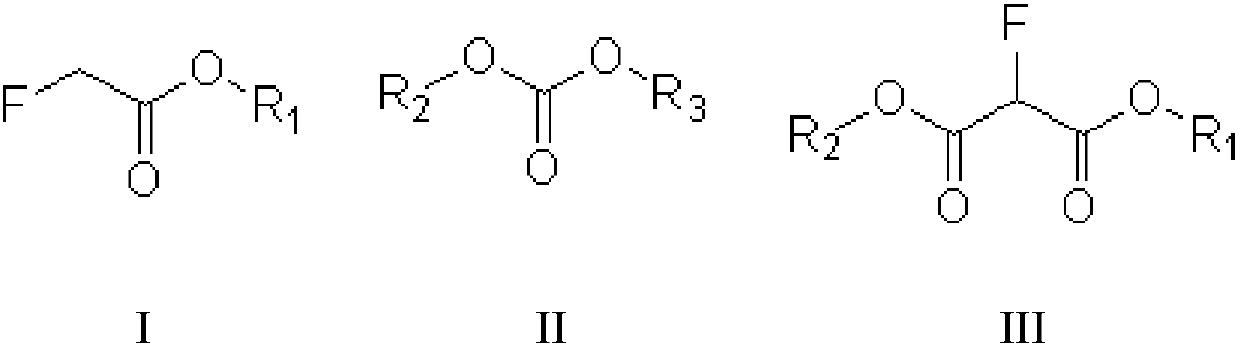
[0027] The invention provides a kind of preparation method of fluoromalonate diester (formula III compound), and described preparation method comprises:
[0028] Condensation reaction of fluoroacetate (compound of formula I) and dialkyl carbonate (compound of formula II) in the presence of alkali to prepare fluoromalonate diester, said formula I, formula II, The structural formula of the compound of formula III is as follows:
[0029]
[0030] In the preparation method provided by the present invention, R1, R2, and R3 can be independently selected from alkyl groups, more specifically, for example, C1~C6 alkyl groups, C1~C3 alkyl groups, etc. In a specific embodiment of the invention, The R1, R2 and R3 may be independently selected from methyl or ethyl. In another specific embodiment of the present invention, the groups of R1, R2 and R3 are the same.
[0031] In the preparation method provided by the present invention, the base can generally provide alkaline conditions in ...
Embodiment 1
[0041] Preparation of dimethyl fluoromalonate:
[0042] In a 250ml round bottom flask, add 80.0g dimethyl carbonate and 5.4g sodium methoxide, heat to reflux, add 9.0g methyl fluoroacetate dropwise, and react for 2h. Use 30% hydrochloric acid to adjust PH=6~7 to quench the reaction, separate the liquid, and distill the organic phase under reduced pressure to obtain 8.3 g of dimethyl fluoromalonate (content 96.5%), with a yield of 54.6%.
Embodiment 2
[0044] Preparation of dimethyl fluoromalonate:
[0045] In a 250ml round bottom flask, add 40g tetrahydrofuran, 40.0g dimethyl carbonate and 10.8g sodium methoxide, heat to reflux, drop 9.0g methyl fluoroacetate, and react for 4h. The reaction was quenched with 30% hydrochloric acid, separated, and the organic phase was distilled under reduced pressure to obtain 8.3 g of dimethyl fluoromalonate (content: 94.2%), with a yield of 53.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com