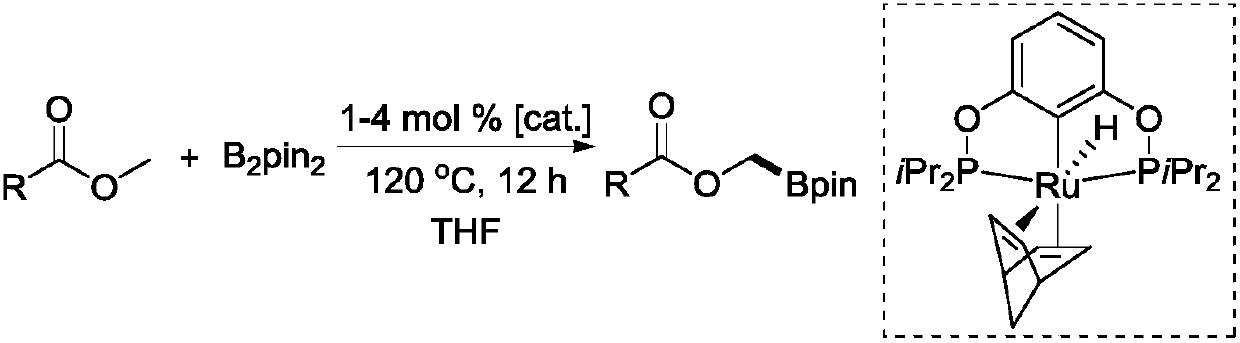
New method for selective dehydrogenation boronation reaction by catalyzing methyl ester through ruthenium
A ruthenium-catalyzed methyl ester and selectivity technology, applied in the field of ruthenium-catalyzed selective dehydroboration of methyl ester derivatives, can solve problems that have not yet been discovered
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
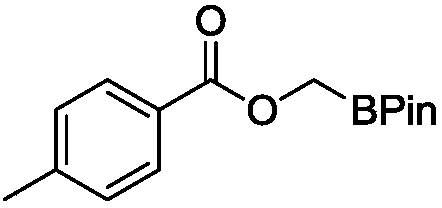
[0019] Embodiment 1, wherein methyl ester substrate is as follows:
[0020] Structural formula of methyl ester substrate:
[0021]
[0022] Add ruthenium complex (3.0 mg, 5.6 μmol), ester substrate (0.56 mmol), B 2 pin 2 (0.14g, 0.56mmol) and 1mL tetrahydrofuran. Then the 5 mL sealed tube was tightened and removed from the glove box and placed in an oil bath at 120° C. for heating and stirring for 12 hours. When the reaction solution was cooled to room temperature, the reaction was quenched with ethyl acetate, and then the low-boiling point organic matter was sucked dry on a rotary evaporator. Finally, methylene bromide in an equimolar amount to the substrate was added as an internal standard to measure the NMR yield of the product. After obtaining the NMR yield, the low-boiling organic matter in the crude product was extracted again, and finally, it was separated and purified by column separation. Colorless transparent oil, the eluent used for passing through the colum...
Embodiment 2
[0023] Embodiment 2, wherein methyl ester substrate is as follows:
[0024] Structural formula of methyl ester substrate:
[0025]
[0026] Add ruthenium complex (3.0 mg, 5.6 μmol), ester substrate (0.56 mmol), B 2 pin 2 (0.14g, 0.56mmol) and 1mL tetrahydrofuran. Then the 5 mL sealed tube was tightened and removed from the glove box and placed in an oil bath at 120° C. for heating and stirring for 12 hours. When the reaction solution was cooled to room temperature, the reaction was quenched with ethyl acetate, and then the low-boiling point organic matter was sucked dry on a rotary evaporator. Finally, methylene bromide in an equimolar amount to the substrate was added as an internal standard to measure the NMR yield of the product. After obtaining the NMR yield, the low-boiling organic matter in the crude product was extracted again, and finally, it was separated and purified by column separation. Colorless transparent oil, the eluent used for passing through the colum...
Embodiment 3
[0027] Embodiment 3, wherein methyl ester substrate is as follows:
[0028] Structural formula of methyl ester substrate:
[0029]
[0030] Add ruthenium complex (3.0 mg, 5.6 μmol), ester substrate (0.56 mmol), B 2 pin 2 (0.14g, 0.56mmol) and 1mL tetrahydrofuran. Then the 5 mL sealed tube was tightened and removed from the glove box and placed in an oil bath at 120° C. for heating and stirring for 12 hours. When the reaction solution was cooled to room temperature, the reaction was quenched with ethyl acetate, and then the low-boiling point organic matter was sucked dry on a rotary evaporator. Finally, methylene bromide in an equimolar amount to the substrate was added as an internal standard to measure the NMR yield of the product. After obtaining the NMR yield, the low-boiling organic matter in the crude product was extracted again, and finally, it was separated and purified by column separation. Colorless transparent oil, the eluent used for passing through the colum...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com