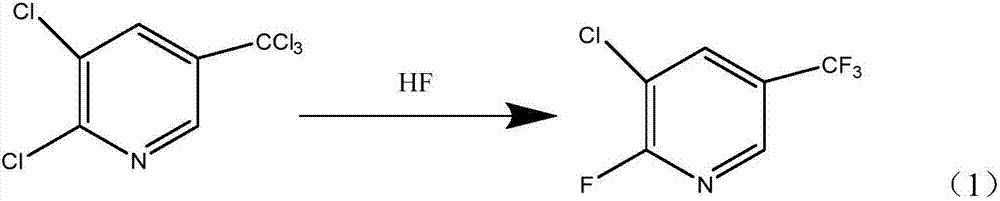
Preparation method of 2-fluoro-3-chloro-5-(trifluoromethyl)pyridine
A technology for trifluoromethylpyridine and crude trifluoromethylpyridine is applied in the field of preparation of 2-fluoro-3-chloro-5 trifluoromethylpyridine, and can solve the problems of difficult separation, serious pollution, high toxicity and the like , to achieve the effect of improving product purity, simple post-processing and low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] 1. In a 200 mL autoclave, add 23.0 g (0.1 mol) of 2,3,-dichloro-5-trichloromethylpyridine, close the lid, fill with nitrogen gas at 10.0 MPa to maintain the pressure for 5 hours, and test the reactor for leaks. After confirming that there is no gas leakage in the reaction kettle, vent the pressure in the kettle. Then the reaction kettle was placed in an ice-salt bath for cooling, when the temperature in the kettle dropped to below -5°C, anhydrous HF40.0g (~2.0mol) was filled into the kettle, and the reaction system was heated to 180 under stirring conditions. ℃, incubated for 12h.
[0035] 2. After the reaction is completed, the temperature is lowered to 25°C, and the reaction kettle is replaced with nitrogen for half an hour (the replaced gas is passed into a 10% aqueous sodium hydroxide solution for neutralization and absorption), and the reaction pressure is poured into a 250mL round-bottomed flask. , 70 g (~1.0 mol) of 25% ammonia solution was added, and the reacti...
Embodiment 2
[0038] The temperature of the reaction system in Example 1 was changed to 200° C., and other operating conditions including post-treatment steps were the same as those in Example 1. 15.6 g of 2-fluoro-3-chloro-5-trifluoromethylpyridine product was obtained, and the content of 2-fluoro-3-chloro-5-trifluoromethylpyridine in the product was 98.65% (GC) by analysis, Yield 95.12%.
Embodiment 3
[0040] The ammoniation reaction temperature in Example 1 was changed to 50° C., and other operating conditions including post-treatment steps were the same as those in Example 1. 14.8 g of 2-fluoro-3-chloro-5-trifluoromethylpyridine product was obtained, and the content of 2-fluoro-3-chloro-5-trifluoromethylpyridine in the product was 98.88% (GC) by analysis, Yield 90.24%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com