Felodipine sustained-release tablet
A gentle and non-alcoholic technology, applied in the field of medicine, can solve the problems of non-constant release rate, not close to constant rate, and not stable blood pressure, so as to improve compliance, uniform release rate and reduce blood pressure fluctuations Effect
Inactive Publication Date: 2018-05-04
SOUTHWEST MEDICAL UNIVERISTY +1
View PDF2 Cites 2 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
However, the sustained-release tablets currently available in the market have the problem that the release rate is not constant or not close to the constant rate, which leads to insufficient blood pressure reduction and reduces the clinical application effect of felodipine sustained-release tablets.
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
[0021]
[0022]
Embodiment 2
[0024]
Embodiment 3
[0026]
[0027]
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
| Property | Measurement | Unit |
|---|---|---|
| viscosity | aaaaa | aaaaa |
Login to View More
Abstract
The invention belongs to the technical field of drugs, and relates to a felodipine sustained-release tablet. The felodipine sustained-release tablet is prepared from the following components in weightby percent: 1 to 5% of felodipine, 5 to 20% of hydroxypropyl methylcellulose, 1 to 10% of hydroxypropyl cellulose, 10 to 40% of hydrophobic fillers, 40 to 70% of hydrophilic fillers, and 5 to 20% ofother pharmaceutical excipients. The felodipine sustained-release tablet can make release degree of the drugs more uniform at a constant speed.
Description
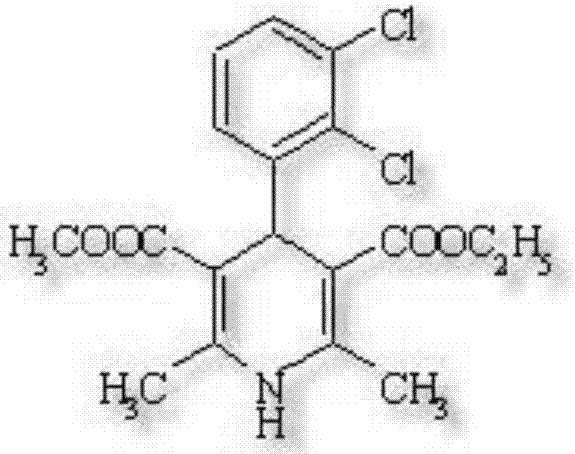
technical field [0001] The invention belongs to the technical field of medicines, in particular to a felodipine slow-release tablet. Background technique [0002] Felodipine, chemical name is (±)-2,6-Dimethyl-4-(2,3-dichlorophenyl)-1,4-dihydro-3,5-pyridinedicarboxylate ethyl ester , the English name is FELODIPINE, the molecular formula is C18H19Cl2NO4, the molecular weight is 384.25, and the chemical structure is as follows: [0003] [0004] It is white to light yellow crystal or crystalline powder; odorless, tasteless; unstable when exposed to light; easily soluble in acetone, methanol or ethanol, almost insoluble in water. [0005] Felodipine is a new type of dihydropyridine calcium channel blocker with a high degree of vascular selectivity, with a selectivity ratio of 100 for blood vessels and heart. Lowers arterial pressure by lowering peripheral vascular resistance, has no direct effect on myocardial contractility and cardiac conduction within the therapeutic dose...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Applications(China)
IPC IPC(8): A61K9/22A61K31/4422A61K47/38A61P9/12
Inventor 赵领魏郁梦皮超刘浩胡美郑文武叶云邓以平
Owner SOUTHWEST MEDICAL UNIVERISTY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com