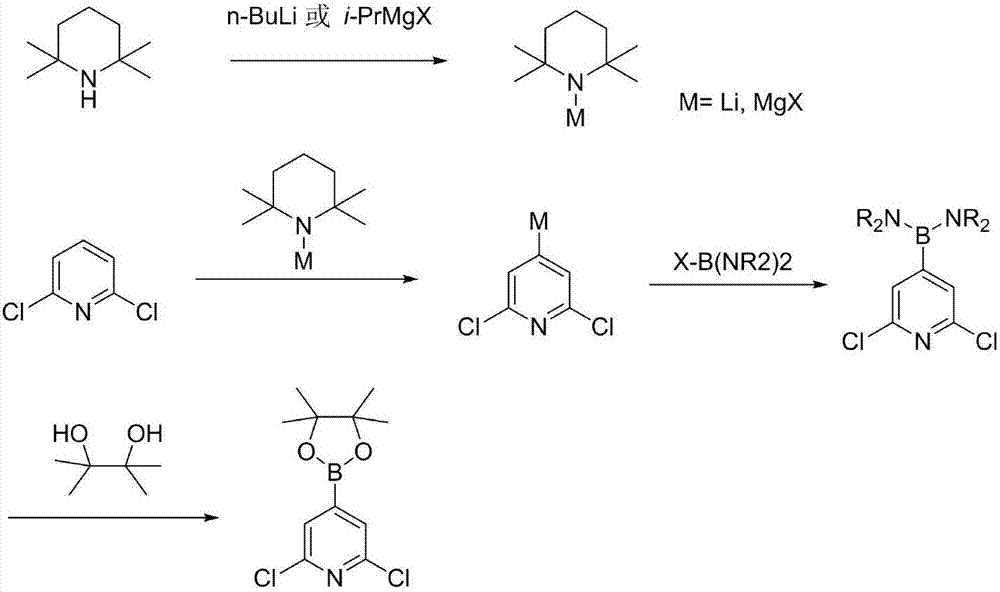
Synthesis process of 2,6-dichloropyridine-4-boracic acid pinacol ester
A kind of technology of dichloropyridine and synthesis process, applied in the field of pharmaceutical intermediate synthesis, can solve problems such as expensive metal iridium catalyst, and achieve the effects of easy recycling and short operation steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Under the protection of nitrogen, 120 ml of anhydrous tetrahydrofuran and 2,2,6,6-tetramethylpiperidine (33.9 g, 0.24 mol) were added to the reaction flask equipped with a dropping device, cooled to -70 ° C to - At 80°C, 96 ml (0.24 mol) of 2.5M n-butyllithium solution was added dropwise. After completion of the dropwise addition, keep stirring for 1 hour. Dissolve 2,6-dichloropyridine (29.6 g, 0.20 mol) in 150 ml of anhydrous tetrahydrofuran, mix well, transfer to the above-mentioned dropping funnel, start adding the mixed solution dropwise, and keep the reaction temperature during the dropping at -70°C to -60°C.
[0019] After the dropwise addition is completed, continue to stir and react for 1-2 hours. After sampling and adding D2O for derivatization, it is confirmed that the conversion rate is greater than 95% (position selectivity 4-position / 3-position 35:1). Then, a mixed solution of BrB(C4H8N)2 (48.5 g, 0.21 mol) dissolved in 180 ml of anhydrous tetrahydrofuran...
Embodiment 2
[0022] Under the protection of nitrogen, 120 ml of anhydrous 2-methyltetrahydrofuran and 2,2,6,6-tetramethylpiperidine (33.9 g, 0.24 mol) were added to the reaction flask equipped with a dropping device, cooled to - From 70°C to -80°C, 96 ml (0.24 mol) of 2.5M n-butyllithium solution was added dropwise. After completion of the dropwise addition, keep stirring for 1 hour. Dissolve 2,6-dichloropyridine (29.6 g, 0.20 mol) in 130 ml of anhydrous 2-methyltetrahydrofuran, mix well and transfer it into the above-mentioned dropping funnel, start adding the mixed solution dropwise, during the dropping process , keep the reaction temperature at -70°C to -60°C.
[0023] After the dropwise addition, continue to stir the reaction for 1-2 hours, take a sample and add D2O to derivatize and confirm that the conversion rate is greater than 95% (position selectivity 4-position / 3-position 33:1). Then in the dropping funnel, add ClB(NMe2) 2 (95% purity, 33.9 grams, 0.24 mole) is dissolved in th...
Embodiment 3
[0026] Under the protection of nitrogen, 120 ml of anhydrous diethoxymethane and 2,2,6,6-tetramethylpiperidine (33.9 g, 0.24 mol) were added to the reaction flask equipped with a dropping device, cooled to - From 70°C to -80°C, 115ml (2M, 0.23mol) of isopropylmagnesium chloride solution was started to be added dropwise. After completion of the dropwise addition, keep stirring for 1 hour. Dissolve 2,6-dichloropyridine (29.6 g, 0.20 mol) in 180 ml of anhydrous diethoxymethane, mix well and transfer it into the above-mentioned dropping funnel, start adding the mixed solution dropwise, during the dropping process , keep the reaction temperature at -70°C to -60°C.
[0027] After the dropwise addition is completed, continue to stir and react for 1-2 hours. After sampling and adding D2O for derivatization, it is confirmed that the conversion rate is greater than 92% (position selectivity 4-position / 3-position 28:1). Then add BrB (NiPr2) 2 (58.2 grams, 0.20 moles) in the dropping fu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com