Preparation method and application of medical porous Al(OH)3 adjuvant
An adjuvant, double-distilled water technology, applied in the field of medical materials and chemistry, can solve the problems of difficulty in determining the exact dose, affecting the quality and reproducibility of animal models of allergic diseases, and not having the prospect of clinical use, and achieving stable adsorption. effect of effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1 Porous Al(OH) 3 Adjuvant preparation
[0028] Anhydrous Al 2 (SO 4 ) 3 Dissolve in 100mL double-distilled water, slowly add 25% NH 3 ·H 2 O, control the dripping time for not less than 6.5 hours, during which the temperature of the reaction system should not exceed 40°C. Waiting for NH 3 ·H 2 After all the O is added dropwise, stir at room temperature for 24h, and then stand and age for 12h to obtain white Al(OH) 3 Sol. The resulting reaction product was repeatedly washed with double distilled water until there was no SO 4 2- can be detected. Then wash 3 times with 50 mL of absolute ethanol, each washing time is 10 min. Centrifuge, discard the supernatant, and store at room temperature with P 2 o 5 Vacuum dry for 48 hours as a desiccant to obtain white solid Al(OH) 3 . Then the obtained Al(OH) 3 The solid was put into an ethylene oxide sterilizer and sterilized for 4 hours. White solid Al(OH) after sterilization 3 Soak in 50mL spectroscopica...
Embodiment 2
[0029] Example 2 Porous Al(OH) 3 Scanning electron microscope (SEM) image of adjuvant
[0030] Such as figure 1 Shown: Al(OH) prepared by the above method 3 Under the field emission scanning electron microscope of the adjuvant, it can be seen that dense holes with different calibers exist. These holes show that a kind of Al(OH) with porous structure prepared by the present invention 3 adjuvant. At the same time, the porous structure provides sufficient space for accommodating specific antigens.
Embodiment 3
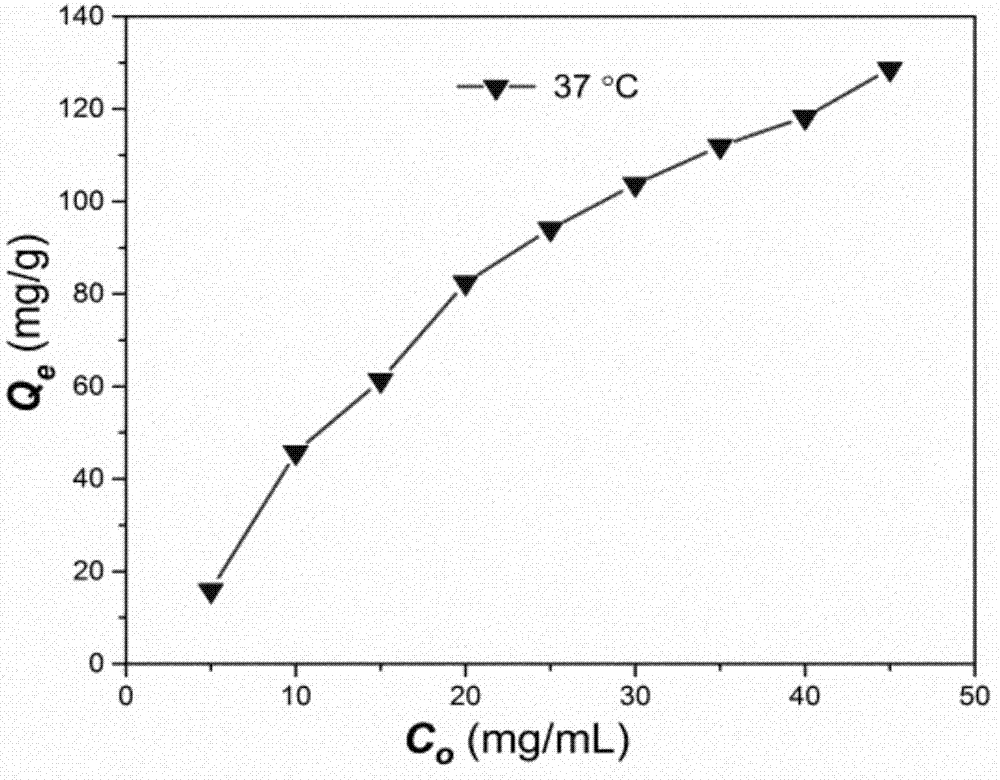
[0031] Example 3Al(OH) 3 Determination of the adsorption amount of adjuvant to specific antigen OVA
[0032] According to the method reported in the literature, the Al(OH) 3 Adjuvant adsorption to specific antigen OVA at body temperature. The result is as figure 2 Shown: The maximum adsorption capacity is 130mg / g.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com