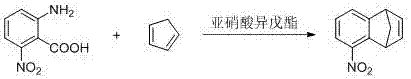
Method and device for continuously preparing 5-nitro-1,4-dihydro-1,4-methano-naphthalene through channelization
A bridge methylene, pipeline technology, applied in chemical instruments and methods, preparation of organic compounds, organic chemistry and other directions, can solve the problems of high risk, unsuitable for industrial production, etc., achieve convenient operation, overcome local concentration inconsistencies Uniform, easy post-processing results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
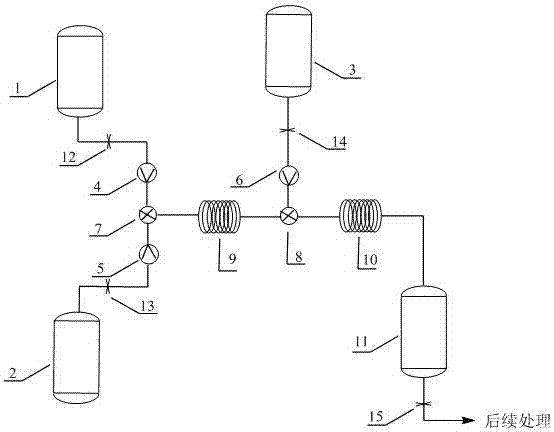
[0026] The structure of the reaction device is as figure 1 , Tubular reactor I 9 in this example: single tube, tube length 5 m, tube diameter 1 mm; tube reactor II 10: single tube, tube length 10 m, tube diameter 1 mm.
[0027] The molar flow ratio of cyclopentadiene, isoamyl nitrite and 2-amino-6-nitrobenzoic acid is 2: 1:1;
[0028] Pre-prepared 2-amino-6-nitrobenzoic acid solution: 2-amino-6-nitrobenzoic acid (1.82Kg, 10mol), diethylene glycol dimethyl ether 9.5Kg.
[0029] Pre-prepared nitrite solution: isoamyl nitrite (1.17Kg, 10mol), trichloroacetic acid (1.62Kg, 10mol).
[0030] Prepare cyclopentadiene: cyclopentadiene (1.32Kg, 20mol).
[0031]Cool the tubular reactor I 9 to 10°C and keep it warm, store the 2-amino-6-nitrobenzoic acid solution and the nitrite solution in the storage tank I 1 and the storage tank II 2 respectively, and store the cyclopentadiene In the reservoir III 3, the 2-amino-6-nitrobenzoic acid solution and the nitrite solution are continuously i...
Embodiment 2
[0033] The structure of the reaction device is as figure 1 , Tubular reactor I 9: single tube, tube length 5 m, tube diameter 3 mm, tube reactor II 10: single tube, tube length 20 m, tube diameter 3 mm.
[0034] The molar flow ratio of cyclopentadiene to 2-amino-6-nitrobenzoic acid is 5:1, the reaction temperature in tubular reactor I9 is 5°C, and the retention time is 60s. The dwell time in 10 is 10s,
[0035] Other operations were the same as in Example 1, and 1.06 Kg of 5-nitro-1,4-dihydro-1,4-endomethylene-naphthalene was finally obtained, with a yield of 57% and a GC content of 92.4%. Gas phase detection conditions are the same as in Example 1.
Embodiment 3
[0037] The structure of the reaction device is as figure 1 , Tubular reactor I 9: single tube, tube length 5 m, tube diameter 3 mm, tube reactor II 10: single tube, tube length 1 m, tube diameter 6 mm.
[0038] The molar flow ratio of cyclopentadiene to 2-amino-6-nitrobenzoic acid is 5:1, the reaction temperature in tubular reactor I9 is -10°C, and the retention time is 60s. In tubular reactor II10 The reaction temperature is 120°C and the residence time is 180s.
[0039] Other operations were the same as in Example 1, and 0.89 Kg of 5-nitro-1,4-dihydro-1,4-endomethylene-naphthalene was finally obtained, with a yield of 48% and a GC content of 95.3%. Gas phase detection conditions are the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com