Gallic acid sulfanilamide derivatives and application thereof in anti-liver cancer
A galloyl sulfonamide and galloyl sulfadiazine technology, applied in the field of gallic acid sulfonamide derivatives and their application in anti-liver cancer, can solve the problem of insufficient anti-cancer research and achieve the effect of expanding the scope of research and development
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
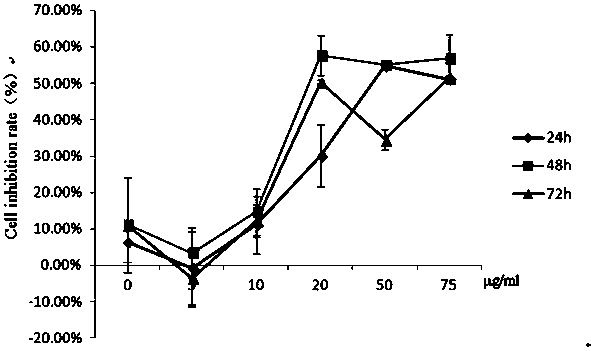
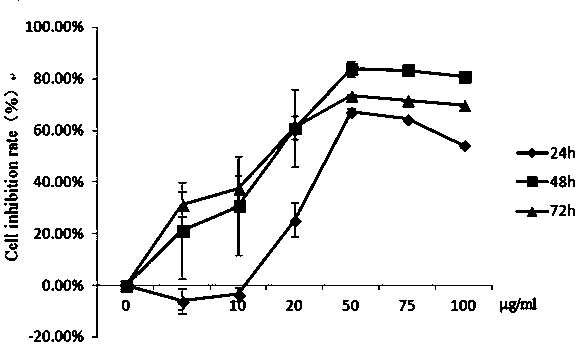
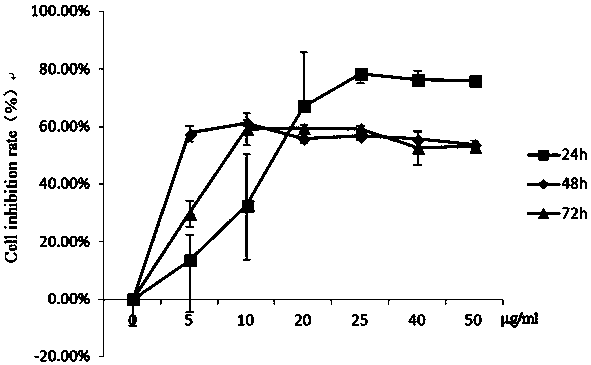
[0034] Example 1: Determination of the killing effect of gallic acid sulfonamide derivative series compounds on mouse liver cancer cells.
[0035] The liver cancer cell line (H 22 ) into a single cell suspension, with 10 4 Cells / well were seeded in a 96-well plate at 37°C, 5% CO 2 Incubate in saturated humidity for 24 h. Take out the 96-well plate, add the above-mentioned mother solution of different compounds or PBS 100μl / well, the final concentration is 1.25μg / ml, 2.5μg / ml, 5μg / ml, 12.5μg / ml, 25μg / ml, 50μg / ml, each compound 9 replicate wells were set up for each concentration. At 37°C, 5% CO 2 Saturated humidity, after cultured for 24h, 48h and 72h respectively, the death rate of each induced liver cancer cell was detected by Cck8 method.
[0036] The experimental steps are as follows:
[0037] 1) Add 10 μl Cck8 solution to each well;
[0038] 2) Place at 37°C, 5% CO 2 Incubate at saturated humidity for 4 hours;
[0039] 3) Measure the absorbance (OD) at 450nm wav...
Embodiment 2
[0043] Example 2: Determination of activation of IFN R by EJTC + / + Killing effect of NK cells on mouse liver cancer cells
[0044] 1. Add IFN R + / + NK cells were resuspended with serum-free RPMI 1640 at a concentration of 1 × 10 7 , placed in a six-well plate, 1ml per well. Add NDV (25 HU / ml) and EJTC (50 μg / mL) respectively, co-culture at 37°C for 16 hours to stimulate, and set a blank control group without stimulation. After stimulation, the cells were taken out, centrifuged with serum-free RPMI 1640 medium at 1500 rpm, 4°C for 8 min, and washed twice; counted with trypan blue, resuspended cells in serum-free RPMI 1640 medium and adjusted the number of cells to 1×10 5 / ml. as effector cells.
[0045] 2. CFSE-labeled target cells (Hepa1-6): Add CFSE to the cell suspension (final concentration is 2 μmol / L), pipette gently and place in 5% CO 2 , Incubate in a 37°C incubator for 10 min; take out the cells and add 10 times the volume of cold RPMI 1640 complete culture medi...
Embodiment 3
[0051] Example 3: Activated mouse IFN R + / + Detection of GrB protein expression in NK cells
[0052] (1) Western blotting
[0053] Collect IFN R by centrifugation (300 rpm, 10 min, 4°C) + / + NK cells were washed twice with ice-cold PBS. Add cell lysate (100 μl / l × 10 7 ), whose components are (30mM Tris-Hcl pH 7.5, 120 mM NaCl, 10% glycerol, 1% Triton X-100), resuspend the cell pellet. Add protease inhibitors to lyse the cells, place in an ice bath for 30 min, collect the lysate, and heat in a water bath at 100°C for 5 min to denature the protein. The kit determines the content of total protein in the lysate, and the steps are as follows:
[0054] 1. Prepare an appropriate amount of BCA working solution by adding 50 volumes of Bicinchoninic acid (BCA) reagent A and 1 volume of BCA reagent B (50:1), and mix well;
[0055] 2. Dilute the protein standard with 0.9% NaCl to a final concentration of 0.5mg / ml;
[0056] 3. Add 0, 1, 2, 4, 8, 12, 16, and 20 μl of the standard to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com