Application of a pentapeptide klpgf to inhibit ache activity
A technique of inhibiting activity and adjuvant therapy, applied in the application field of pentapeptide KLPGF inhibiting AChE activity, can solve problems such as complicated pathogenesis and adverse reactions, and achieve the effects of improving nerve conduction, improving dynamic balance, and enhancing memory
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] The measurement of AChE, BChE inhibitory activity of egg white active peptide in vitro of embodiment 1
[0018] 140 μL of sodium phosphate buffer (pH 8.0), 20 μL of test sample solution (50 μg / mL) and 20 μL of ChE / BChE solution were mixed and incubated at room temperature for 15 minutes. Then 10 μL DTNB and 10 μL Ch / BCh were added to the mixture. The absorbance value of each group was detected (20min) by a microplate reader at 412nm.
[0019] Inhibition rate%=(Abs control-Abs sample) / Abs control×100%
[0020] where Abs represents the absorption value.
[0021] The results showed that the peptide KLPGF has good anti-senile dementia activity. When its concentration is 50 μg / mL, the inhibition rate to AChE is 61.23%, and the inhibition rate to BChE is 3.29%.
Embodiment 2
[0022] Example 2 Molecular docking
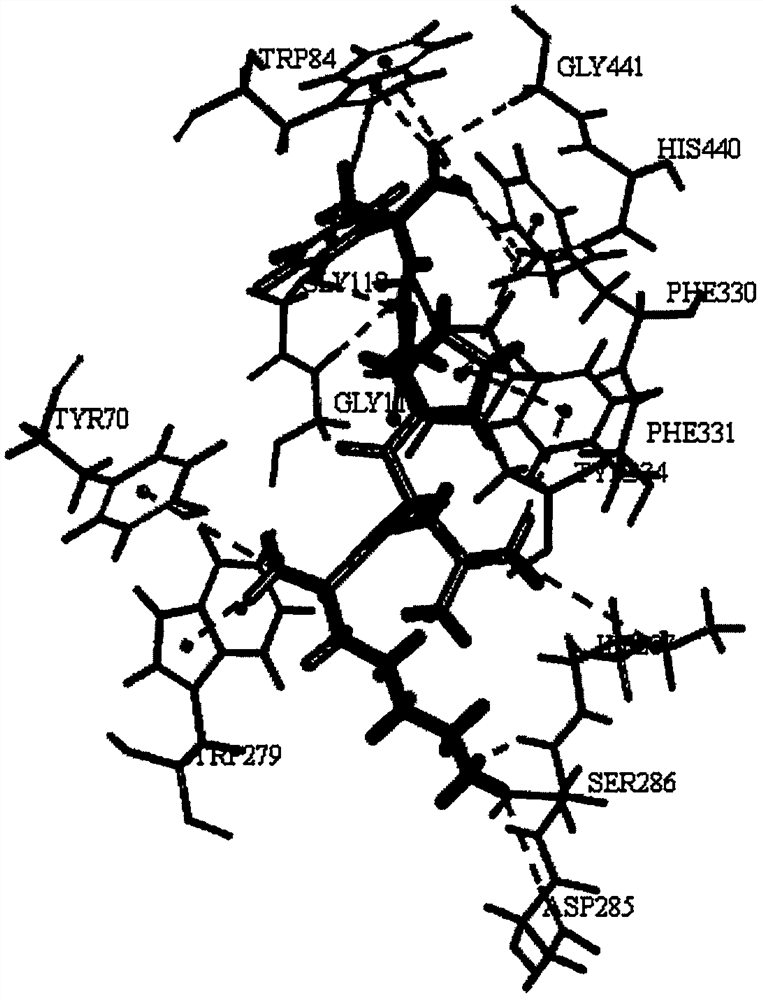
[0023] The crystal structure (1EVE) of AChE was obtained from the PDB database, and it was set as a receptor protein by means of DS, and the active center position was (2.855796, 64.576950, 67.98141). The peptide sequence with the highest activity in step 1 was docked with AChE by CDOCKER, and the parameters were set as follows: Top Hit 10, Pose Cluster Radius 0.1.
[0024] The results showed that the peptide KLPGF could tightly bind to AChE, and the interaction between KLPGF and AChE involved Tyr70-Trp84-Gly118-Gly119-Trp279-Asp285-Ser286-Ile287-Phe330-Phe331-Tyr334-His440-Gly441 amino acid residues. It has strong interaction with the catalytic site of AChE (Tyr70, Trp84, Gly118, Gly119, Trp279, Tyr334, His440). The results of the interaction between peptide KLPGF and AChE are as follows figure 1 shown.
Embodiment 3
[0025] Example 3 Toxicity Prediction
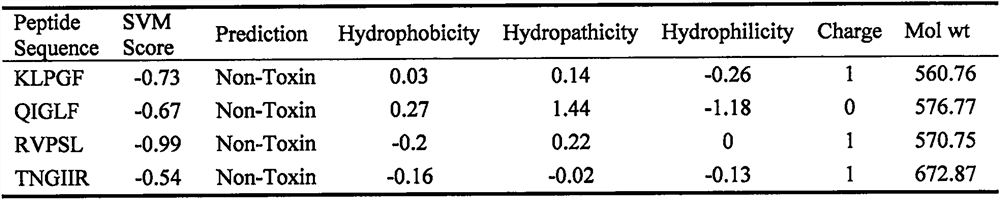
[0026] The potential toxicity of the peptide was predicted by ToxinPred, and SVM score <0 indicated no toxicity. The measurement results are shown in Table 1, and the results showed that the SVM score of the peptide KLPGF was <0, indicating no toxicity.
[0027] Table 1 Toxicity prediction results of egg white peptides
[0028]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com