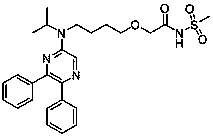
Preparation method of selexipag crystal form
A technology of Siripag and crystal form, which is applied in the field of medicine and can solve problems such as affecting product quality and easy residue of solvents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0011] Embodiment 1: Preparation of cilipag crystal form
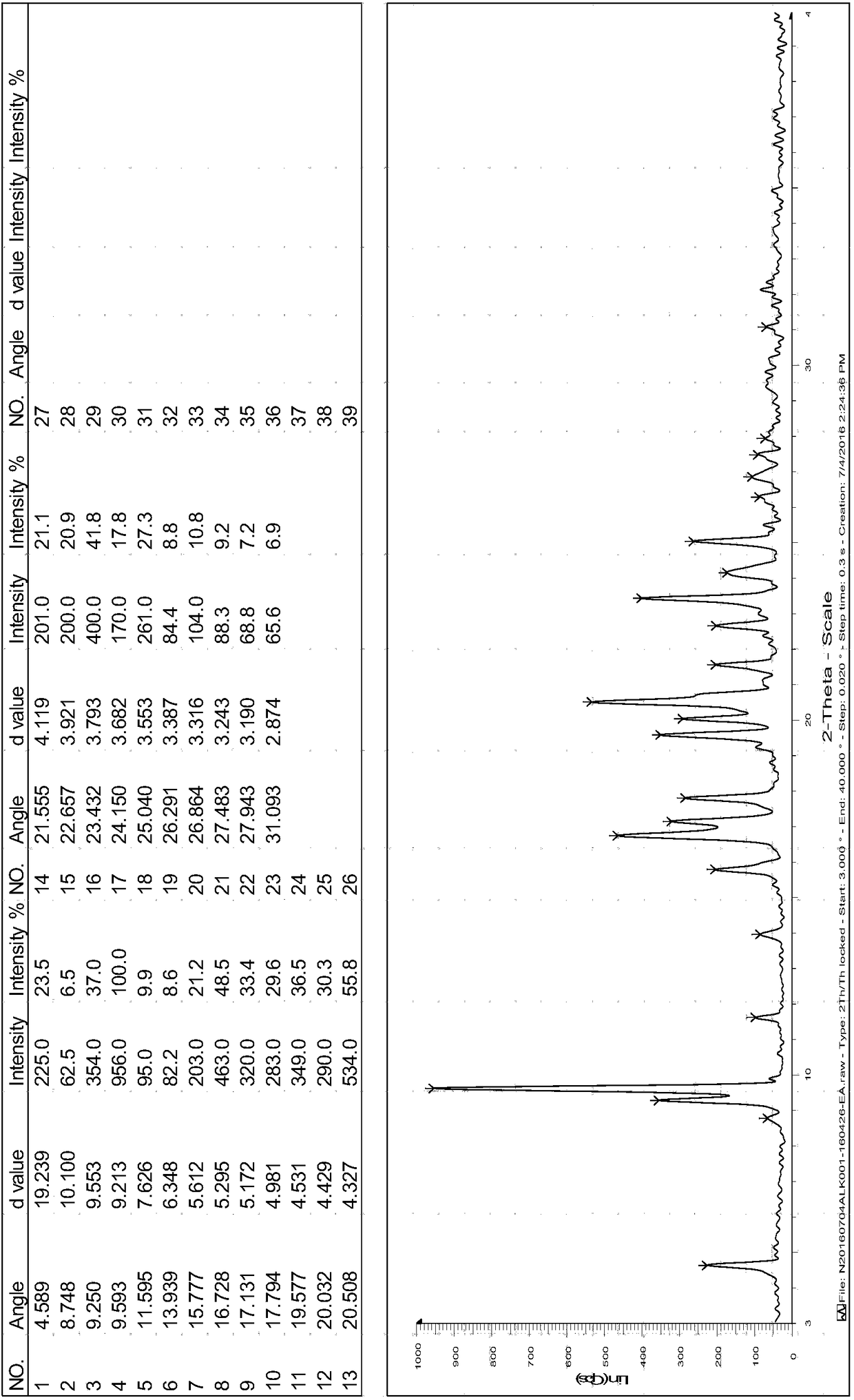
[0012] Add 5g of Silipag and 40ml of ethyl acetate into the reaction flask and continue to stir. The solution gradually dissolves at 75°C~80°C. After the dissolution, cool down to room temperature for crystallization. A large amount of white solids are precipitated in the reaction flask. Keep stirring at -5°C~5°C for 2 hours, filter to obtain a solid, and dry it at 40°C for 4 hours. The residual ethyl acetate is 0.086%, and the HPLC purity is 99.94%. The X-ray powder diffraction pattern is shown in Figure 1 Show:
[0013] The characteristic peaks are as follows:
[0014] 2θ(°)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com