Kit and method for predicting hepatocarcinogenesis risk
A kit and risk technology, which are used in kits and fields for predicting the risk of liver cancer, can solve the problems of low sensitivity of blood free nucleic acid detection and narrowing of application scope.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
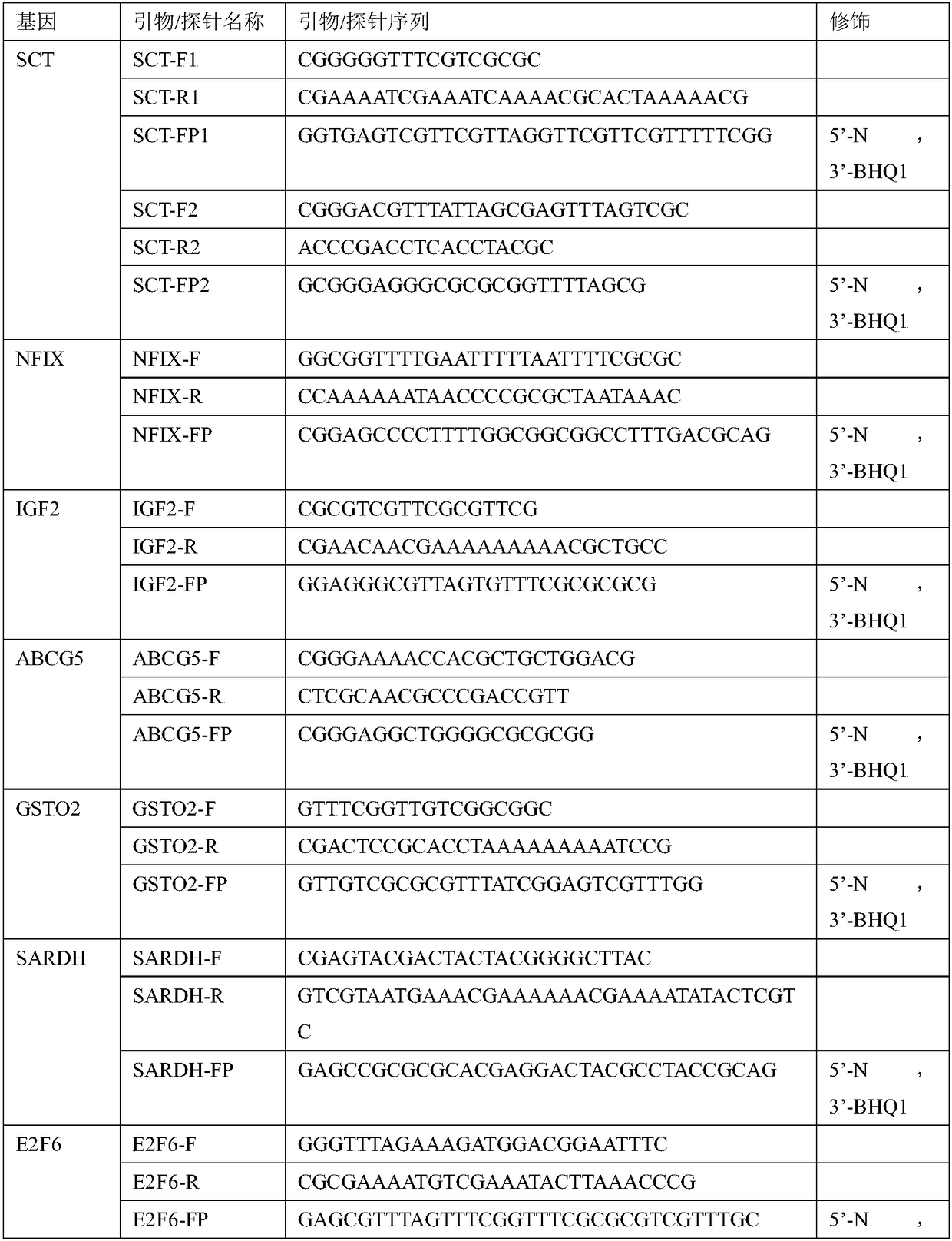
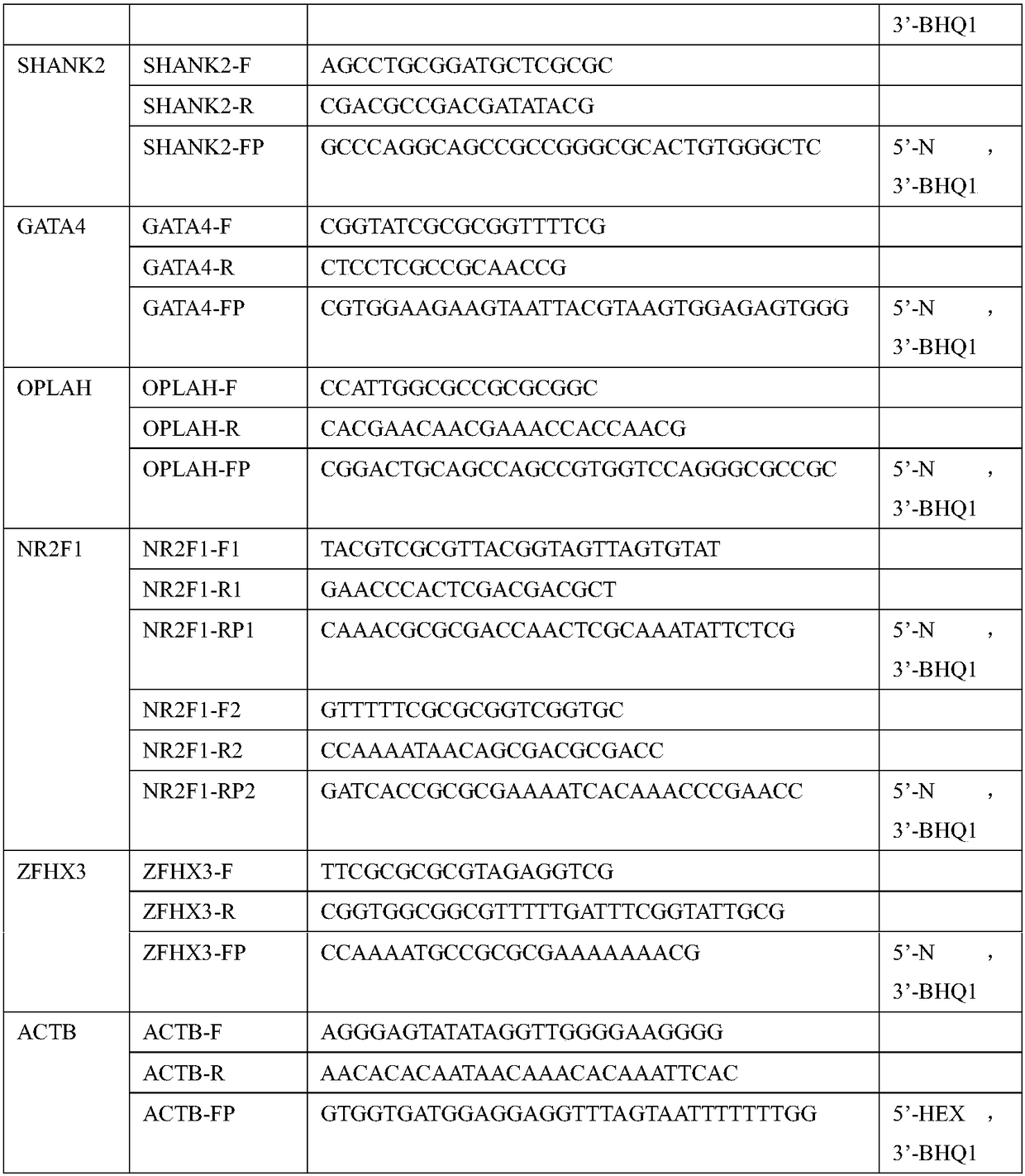
[0075] Example 1 Detection of methylation levels of gene combinations by methylation-specific fluorescent PCR method based on bisulfite conversion
[0076] 1. Collection and preparation of blood samples
[0077] 1. Use cfDNA preservation tubes such as Streck Cell-Free DNA BCT blood collection tubes to collect peripheral blood, and the collected volume is 1-10mL.
[0078] 2. Store at 2-8°C or at room temperature within 7 days.
[0079] 3. Take 2mL of peripheral blood collected in the cfDNA preservation tube, and centrifuge to separate the plasma: centrifuge at 1350±150rcf for 12 minutes.
[0080] 2. Extraction of cfDNA and BS conversion
[0081] 1. Use a peripheral blood free DNA extraction kit such as QIAGEN QIAseq cfDNA Extraction Kit to extract cfDNA from the separated plasma and negative and positive quality controls. The extraction of cfDNA in this method adopts the magnetic bead method to absorb, purify, elute and enrich the cfDNA, and can also use the separation colum...
Embodiment 2
[0097] Example 2 Methylation-binding protein enrichment-fluorescent PCR detection method to detect the methylation level of gene combinations
[0098] 1. Collection and preparation of blood samples
[0099] 1. Use cfDNA preservation tubes such as Streck Cell-Free DNA BCT blood collection tubes to collect peripheral blood, and the collected volume is 1-10mL.
[0100] 2. Store at 2-8°C or at room temperature within 7 days.
[0101] 3. Take 2mL of peripheral blood collected in the cfDNA preservation tube, and centrifuge to separate the plasma: centrifuge at 1350±150rcf for 12 minutes.
[0102] 2. Extraction of cfDNA and capture and enrichment of MBD protein
[0103] 1. Use a peripheral blood cell-free DNA extraction kit such as QIAGEN QIAseq cfDNA Extraction Kit to extract cfDNA from the separated plasma. The extraction of cfDNA in this method adopts the magnetic bead method to absorb, purify, elute and enrich the cfDNA, and the method of separation column can also be used.
...
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com