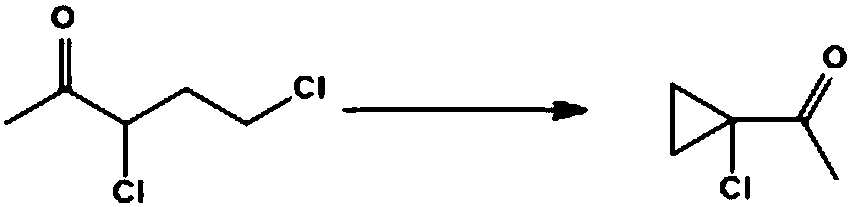Improved method for synthesizing 1-acetyl-1-chlorocyclopropane
A synthesis method, the technology of chlorocyclopropane, which is applied in the field of synthesis of pesticide intermediates, can solve the problems of difficult processing, a large amount of tar, and high prices, and achieve the effects of avoiding environmental pollution, easy operation, and lower production prices
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
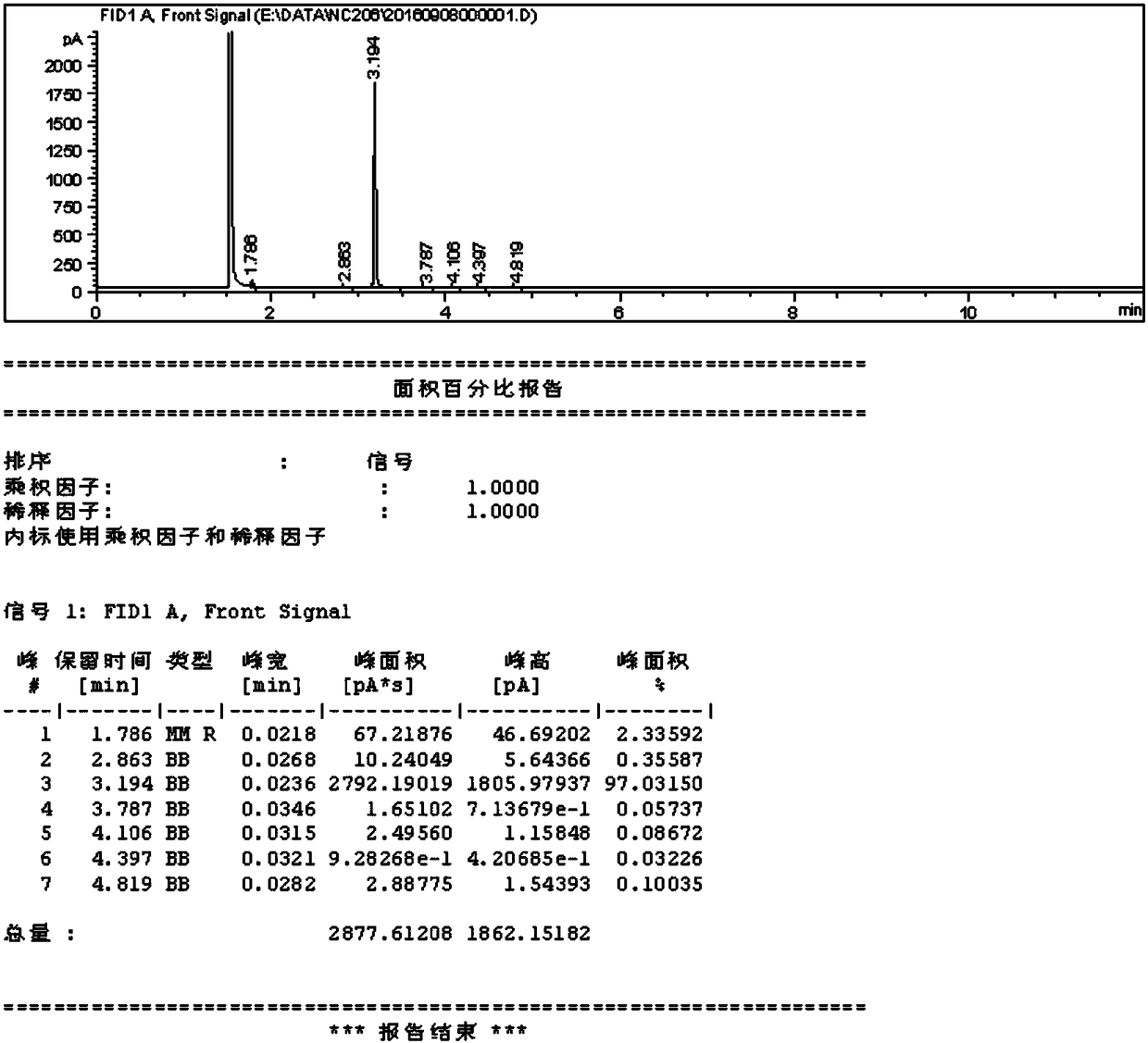
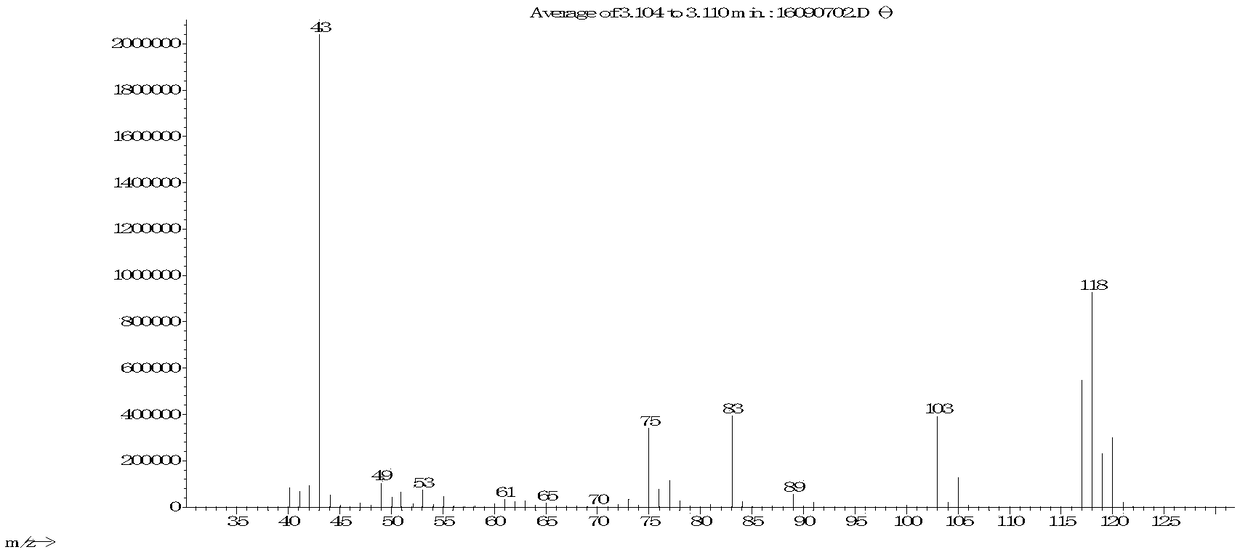
[0025] Add sodium carbonate (0.15mol) and 100g acetonitrile to a 250ml four-neck bottle, start stirring, and add 3,5-dichloro-2-pentanone ( 0.1mol), 10min after dripping, heat preservation reaction for 5-6h, gas phase control detection 3,5-dichloro-2-pentanone < 1%, the reaction is considered complete, the system is cooled to 25 ° C, suction filtration, filtrate rectification Acetonitrile was recovered, and the still residue was continued to rectify to obtain the product (weight 9.8g). The reaction yield was 85.0%, and the isolated yield was 83.0%.
Embodiment 2
[0027] Add sodium bicarbonate (0.3mol) and 100g acetonitrile to a 250ml four-necked bottle, start stirring, heat the system to 80-85°C, add 3,5-dichloro-2-pentanone dropwise at this temperature (80-85°C) (0.1mol), after 10 minutes of dripping, heat preservation reaction for 10 hours, gas phase control, detection of 3,5-dichloro-2-pentanone <1%, as the reaction is complete, the system is cooled to 25 ° C, suction filtration, filtrate rectification Collect the solvent and continue to rectify to obtain the product with a weight of 9.30 g, a reaction yield of 83.1%, and an isolation yield of 80.4%.
Embodiment 3
[0029] In a 250ml four-necked bottle, add sodium carbonate (0.15mol), 100g of N-methylpyrrolidone, start stirring, heat the system at 100-110°C, add 3,5-dichloro-2 -Pentanone (0.1mol), after 10 minutes of dripping, keep warm for 2 hours, control in the gas phase, detect 3,5-dichloro-2-pentanone < 1%, the reaction is considered complete, cool the system to 25°C, filter with suction, The filtrate is rectified to collect solvent and product. The weight is 9.4 g, the reaction yield is 82.8%, and the isolated yield is 79.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com