Electrolyte solution for nonaqueous electrolyte batteries, and nonaqueous electrolyte battery using same
A non-aqueous electrolyte and electrolyte technology, applied in the direction of non-aqueous electrolyte batteries, non-aqueous electrolytes, battery electrodes, etc., can solve the problems of increased cost, increased process, and insufficient effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
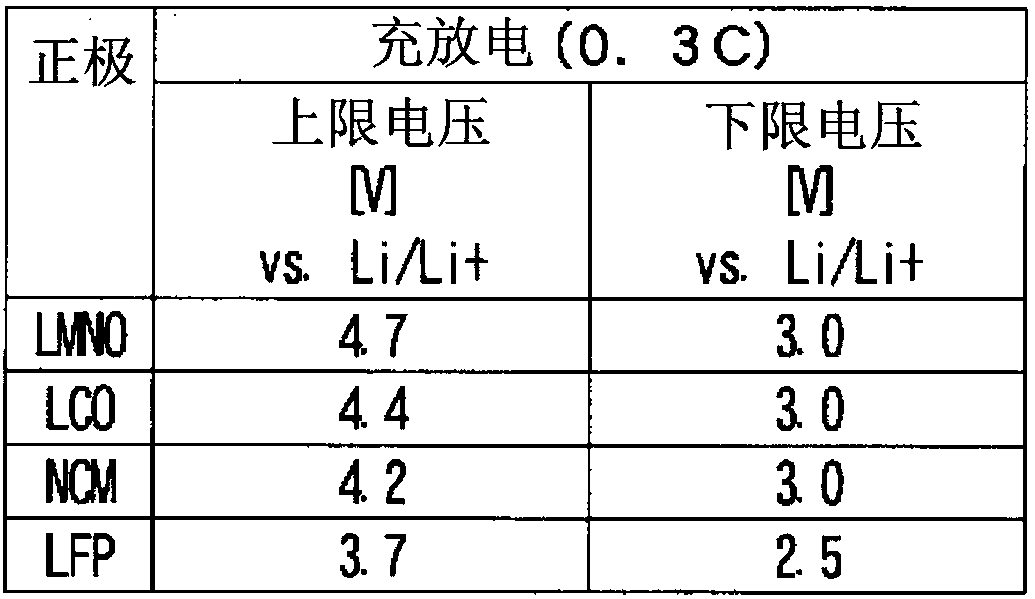
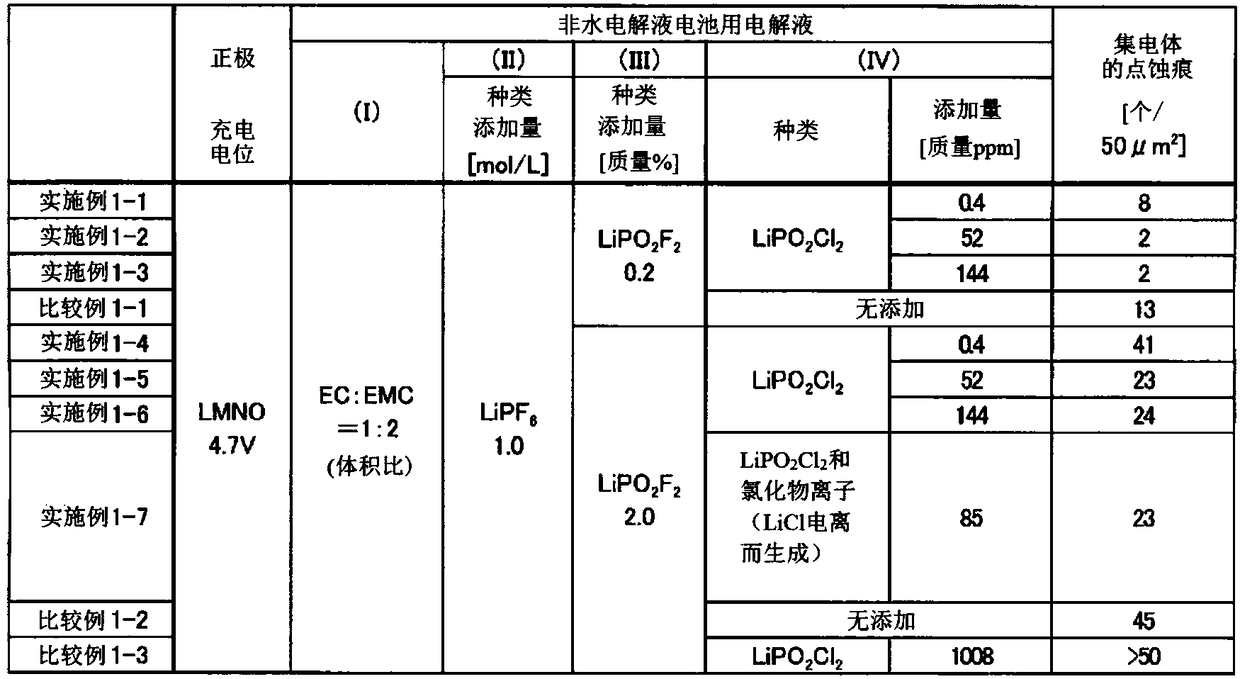
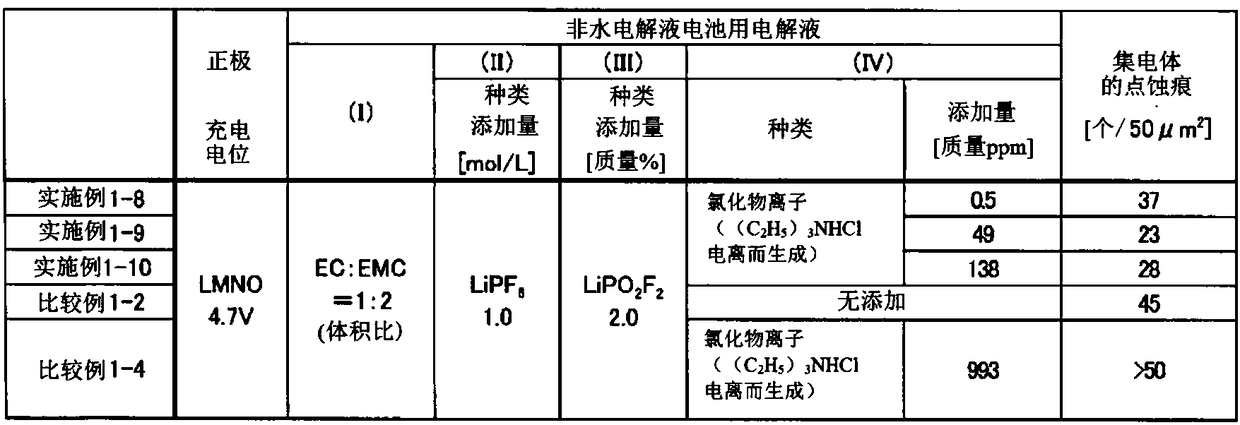
Embodiment 1-1
[0208] Using the nonaqueous electrolytic solution prepared by dissolving lithium difluorophosphate as the component (III) in the test solution 1 at a concentration of 0.2% by mass, a nonaqueous electrolytic solution battery (positive electrode LMNO) was prepared as described above, and then Corrosion evaluation of positive electrode current collector aluminum was performed after initial charge and discharge. The results are shown in Table 2.
Embodiment 1-2
[0210] The corrosion evaluation of the positive electrode current collector aluminum was performed in the same procedure as in Example 1-1 except that the test liquid 2 was used instead of the test liquid 1. The results are shown in Table 2.
Embodiment 1-3
[0212] The corrosion evaluation of the positive electrode current collector aluminum was performed in the same procedure as in Example 1-1, except that the test solution 3 was used instead of the test solution 1. The results are shown in Table 2.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| lattice spacing | aaaaa | aaaaa |
| lattice spacing | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com