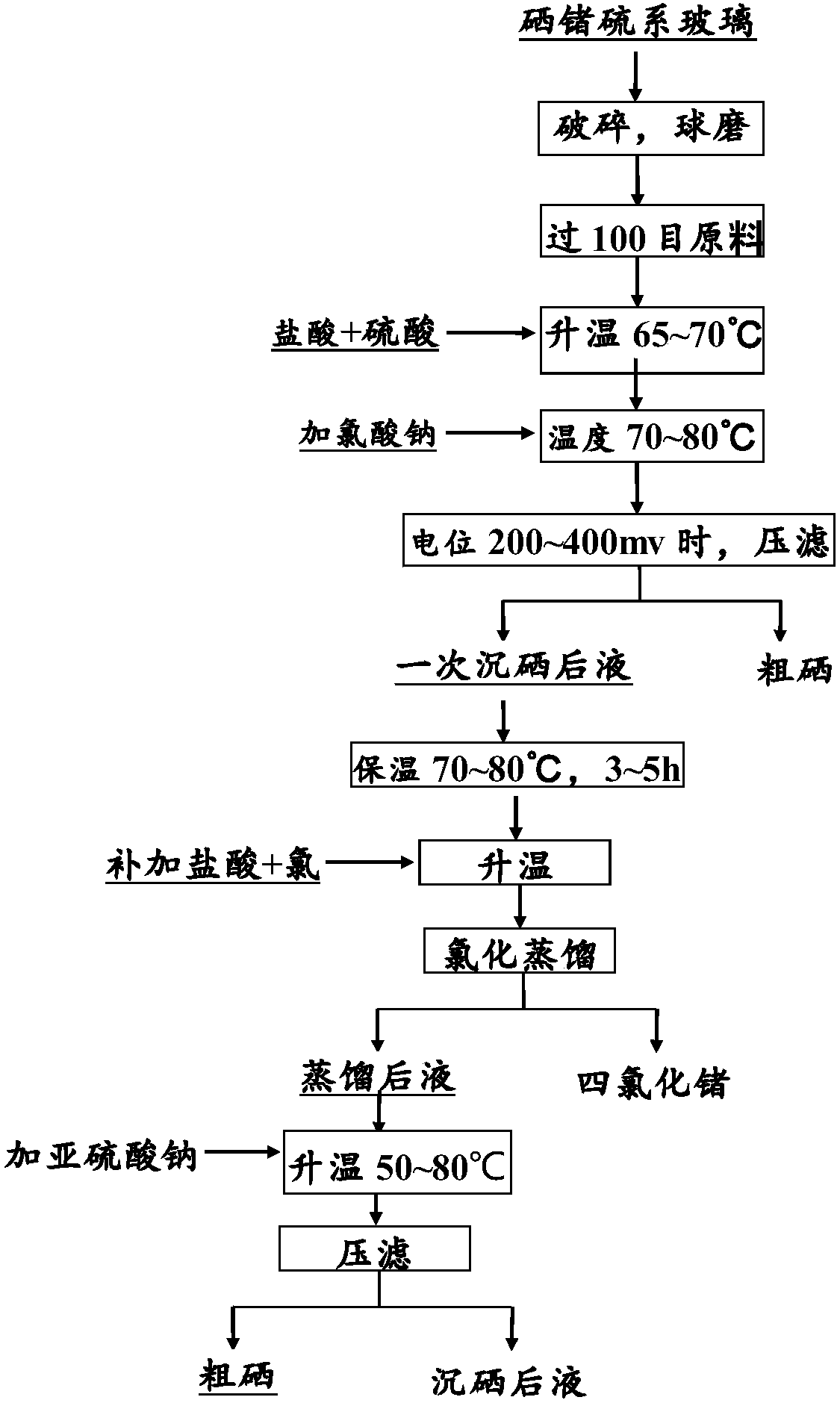
Recovery method of selenium-germanium-sulfur glass
A recovery method and technology of chalcogenide glass, which is applied in the field of hydrometallurgy, can solve the problems affecting germanium grade and difficult recovery of selenium simple substance, etc., and achieve the effect of high recovery rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Selenium germanium chalcogenide glass contains 22% selenium and 12% germanium.
[0042] 700g of material was crushed and ball-milled through a 100-mesh sieve, and 2800ml of 31% hydrochloric acid was added to the reactor, and 700ml of 95% concentrated sulfuric acid was added at a rate of 28L / h. After the addition, start stirring, and then add the sieved material, the mass volume ratio is 1g:5ml. After adding the materials, turn on the heating and raise the temperature to 67°C; add the saturated sodium chlorate solution into the reactor at a rate of 3L / h. During the process of adding, control the reaction temperature at 71°C, and when the potential rises to 221mV, stop adding, cool down, and press filter to obtain the filter residue and the liquid after a selenium precipitation; the filter residue weighs 57g, which is crude selenium with a purity of 93.8%.
[0043] Control the reaction temperature at 79°C and keep it warm for 4h. After the heat preservation finishes, ad...
Embodiment 2
[0046] Selenium germanium chalcogenide glass contains 27% selenium and 19% germanium.
[0047]600g of material was crushed and ball-milled through a 100-mesh sieve, and 3000ml of 32% hydrochloric acid was added to the reactor, and 600ml of 96% concentrated sulfuric acid was added at a rate of 20L / h. After the addition, start stirring, and then add the sieved material, the mass volume ratio is 1g:6ml. After adding the materials, turn on the heating and raise the temperature to 69°C; add the saturated sodium chlorate solution into the reactor at a rate of 5L / h. During the addition, control the reaction temperature at 76°C, and when the potential rises to 277mV , stop adding, lower the temperature, and press filter to obtain a filter residue and a liquid after selenium precipitation; the filter residue is crude selenium with a purity of 97.1%, weighing 41g.
[0048] Control the reaction temperature at 80°C and keep it warm for 5h. After the heat preservation is finished, add 20...
Embodiment 3
[0051] Selenium germanium chalcogenide glass contains 20% selenium and 13% germanium.
[0052] 700g of the material was crushed and ball-milled through a 100-mesh sieve, and 2100ml of hydrochloric acid with a concentration of 32% was added to the reactor, and 700ml of concentrated sulfuric acid with a concentration of 98% was added at a rate of 8L / h. After the addition, start stirring, and then add the sieved material, the mass volume ratio is 1g:4ml. After adding the materials, turn on the heating and raise the temperature to 69°C. Add the saturated solution of sodium chlorate to the reactor at a rate of 5L / h; during the process of adding, control the reaction temperature at 79°C, and when the potential rises to 319mV, stop adding, cool down, and press filter to obtain the filter residue and a precipitate Selenium liquid; the filter residue is crude selenium with a purity of 98.3%, weighing 21g.
[0053] Control the reaction temperature to 80°C and keep it warm for 4h. Aft...
PUM
| Property | Measurement | Unit |
|---|---|---|
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com