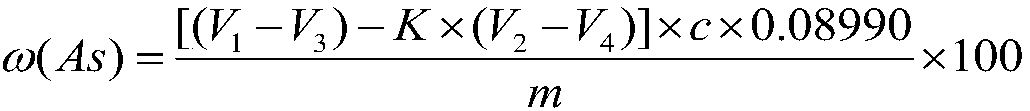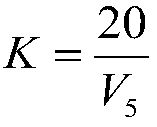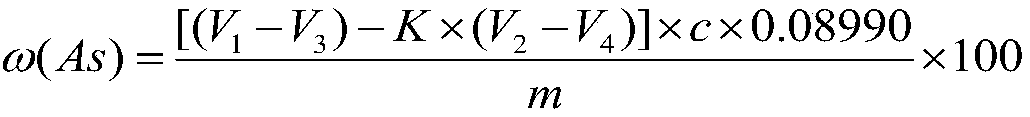Method for determining arsenic content in ore
A technology of ore and content, applied in the field of analytical chemistry, to achieve the effect of high accuracy and wide measurement range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Using GBW 07278 arsenic ore national first-class standard material to carry out experiments, verify the precision and accuracy of the method, a method for measuring arsenic content in ore, the specific steps are as follows:
[0043] Step 1, the ore that particle size is 0.038~0.074mm is pulverized, dried and weighed 0.2000g sample and puts into 500mL conical flask, then adds 15mL concentrated nitric acid;
[0044] Step 2, place the above-mentioned Erlenmeyer flask on the electric stove and heat for 2 minutes;
[0045] Step 3, take off the Erlenmeyer flask from the electric stove, add 0.3g potassium chlorate, then place the Erlenmeyer flask on the electric stove to heat, after the sample is completely dissolved, take it off and cool;
[0046] Step 4, add 10mL (1+1) sulfuric acid to the Erlenmeyer flask, rinse the bottle wall with water, heat to emit sulfur trioxide smoke, keep the smoke state for 3min, remove and cool;
[0047]Step 5. Add 30mL water to the Erlenmeyer fl...
Embodiment 2
[0081] Adopt YSS021-2004 copper concentrate national primary standard material to carry out experiment, verify the precision and accuracy of the method, a kind of method for measuring arsenic content in ore, concrete steps are as follows:
[0082] Step 1, the ore that particle size is 0.038~0.074mm is pulverized, dried and weighed 0.3000g sample and puts into 500mL conical flask, then adds 18mL concentrated nitric acid;
[0083] Step 2, place the above-mentioned Erlenmeyer flask on the electric stove and heat for 3 minutes;
[0084] Step 3, take off the Erlenmeyer flask from the electric stove, add 0.4g potassium chlorate, then place the Erlenmeyer flask on the electric stove to heat, after the sample is completely dissolved, take it off and cool it;
[0085] Step 4, add 12mL (1+1) sulfuric acid into the Erlenmeyer flask, rinse the bottle wall with water, heat to emit thick smoke of sulfur trioxide, keep the state of emitting dense smoke for 4min, take it off and cool;
[008...
Embodiment 3
[0120] Adopt GBW07175 antimony ore national primary standard material to carry out experiment, the precision and accuracy of verification method, a kind of method for measuring arsenic content in ore, concrete steps are as follows:
[0121] Step 1, the ore that particle size is 0.038~0.074mm is pulverized, dried and weighed 0.5000g sample is put into 500mL conical flask, then adds 20mL concentrated nitric acid;
[0122] Step 2, place the above-mentioned Erlenmeyer flask on the electric stove and heat for 5 minutes;
[0123] Step 3, take off the Erlenmeyer flask from the electric stove, add 0.5g potassium chlorate, then place the Erlenmeyer flask on the electric stove to heat, after the sample is completely dissolved, take it off and cool it;
[0124] Step 4, add 15mL (1+1) sulfuric acid in the Erlenmeyer flask, rinse the bottle wall with water, heat to emit thick smoke of sulfur trioxide, keep the state of emitting dense smoke for 5min, take off and cool;
[0125] Step 5. Add...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com