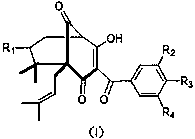
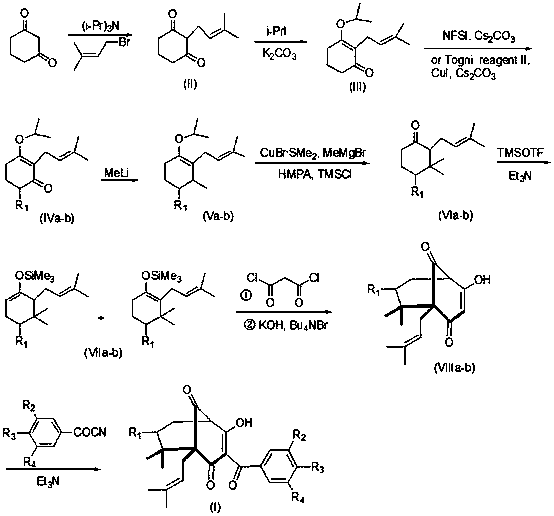
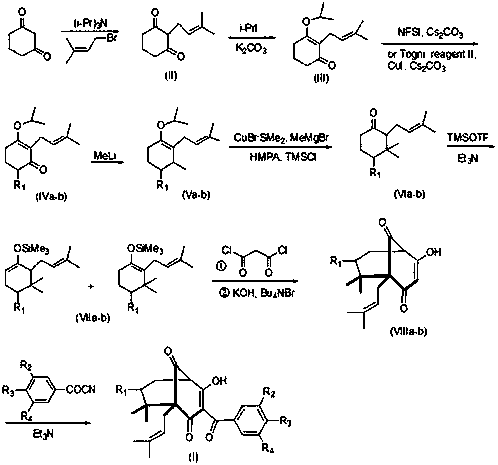
Garcinol derivative and application thereof
A kind of derivative, the technology of mangosteen, applied in mangosteen derivatives and application fields, can solve the problems of mucosal permeability, unsatisfactory bioavailability, etc., and achieve the effect of good prevention and management of cardiac insufficiency and low toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Dissolve 56g (0.5mol) of 1,3-cyclohexanedione in 100ml of water, cool to ℃, slowly add 71.5g (0.5mol) of triisopropylamine, then slowly add 82g (0.55mol) of 3, 3-Dimethylallyl bromide, stirred overnight at room temperature. Add 725 mL of 2N NaOH solution to the reaction solution, separate layers, extract the aqueous phase with 300 ml of ethyl acetate, acidify the aqueous phase with glacial acetic acid to pH 3-4, suction filter, and dry without purification. Intermediate (II) was obtained with a yield of 60%. ESI-MS m / z: 181.0 [M+H].
[0021] Take 35.3g (0.196mol) of intermediate (II) and dissolve it in 300ml of acetone, add 116.6g (0.686mol) of isopropyl iodide and 64g (0.784mol) of potassium carbonate. React overnight. The acetone was distilled off under reduced pressure, and 400ml of water and 400ml of trichloromethane were added to the residue to dissolve. The layers were separated and the aqueous phase was extracted with dichloromethane (2x300ml) and dried. Con...
Embodiment 2
[0029] Take 22.3g (0.1mol) of intermediate (Ⅲ) and dissolve it in 100ml of CHCl 3 In, add 7.6g (0.004mol) CuI and 11.7g (0.11mol) Cs 2 CO 3 , cooled to -10°C, added 34.8g (0.11mol) Togni reagent II, reacted for 24h, filtered, concentrated, and purified by silica gel column chromatography to obtain 3-isopropoxy-2-(3-methyl-2-butane Alkenyl)-6-trifluoromethylcyclohex-2-enone (IVb), 67% yield. ESI-MS m / z: 291.1 [M+H].
[0030] 24g (0.1mol) of intermediate (IVa) was changed to 29g (0.1mol) of intermediate (IVb), and other operations were the same as in Example 1 to obtain intermediate (Vb) with a yield of 71%.
[0031] 12.0 g (50 mmol) of intermediate Va was changed to 14.5 g (50 mmol) of intermediate Vb, and other operations were the same as in Example 1 to obtain intermediate VIb with a yield of 84%.
[0032] 25.4g (100mmol) of intermediate VIa was changed to 26.2g (100mmol) of intermediate VIb, and other operations were the same as in Example 1 to obtain compound VIIIb with...
Embodiment 3
[0035] Change 296mg (1.2mmol) 3,4-diacetoxybenzoyl nitrile into 212mg (1.2mmol) 3-methoxy-4-hydroxybenzoyl nitrile, and other operations are the same as in Example 2 to obtain compound (3 ), yield 75%. ESI-MS m / z: 479.2 [M-H].
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com