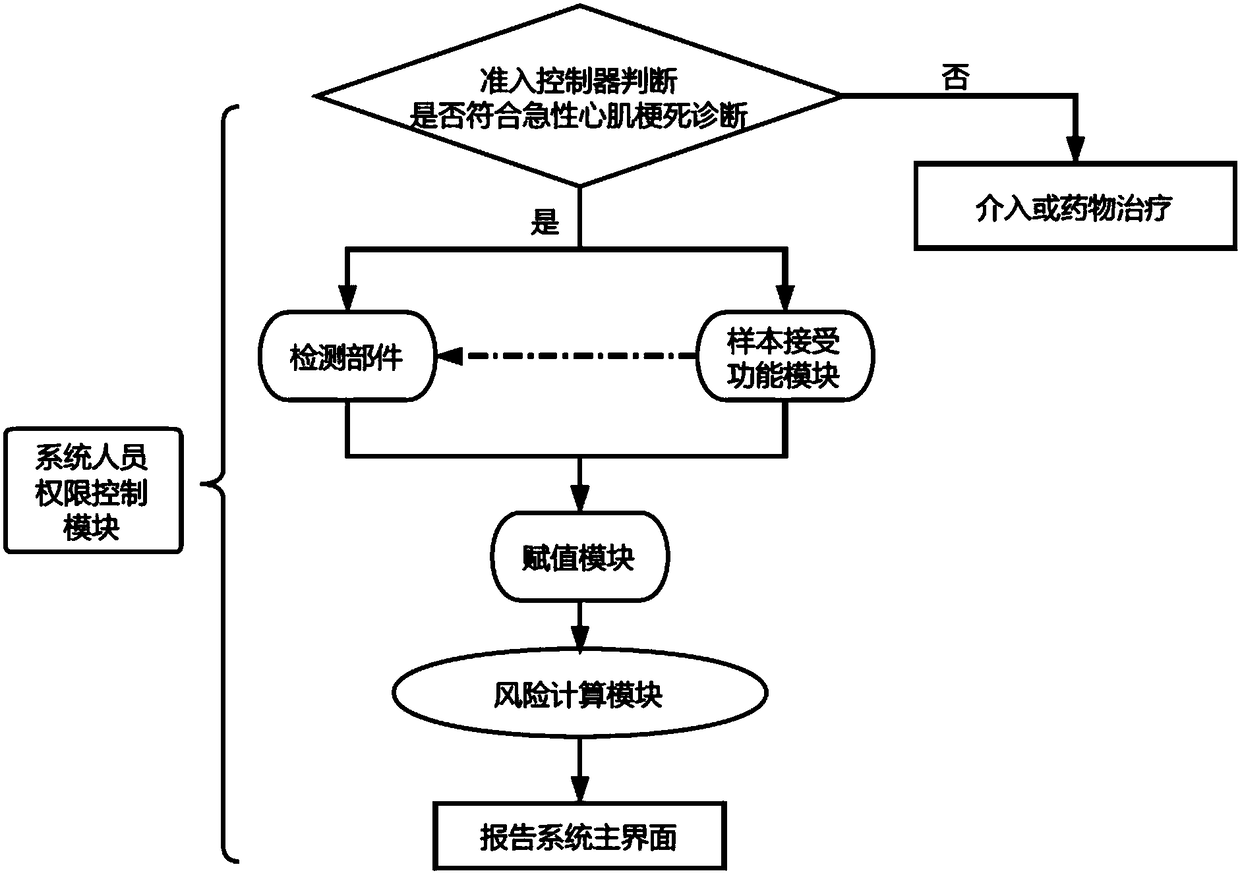
System and kit for predicting adverse events after myocardial infarction
A technology for adverse events and detection components, applied in the field of medical diagnosis, can solve problems such as difficult to standardize the assessment of long-term adverse event risks, large differences in training, and no risk assessment of adverse events in patients with acute myocardial infarction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0117] Example 1 Thyroid-stimulating hormone detection reagent detects adverse events after myocardial infarction
[0118] After the patient confirmed acute myocardial infarction, 2ml of serum was extracted immediately, and the thyroid-stimulating hormone level of the sample was calculated according to the aforementioned thyroid-stimulating hormone detection method of this patent.
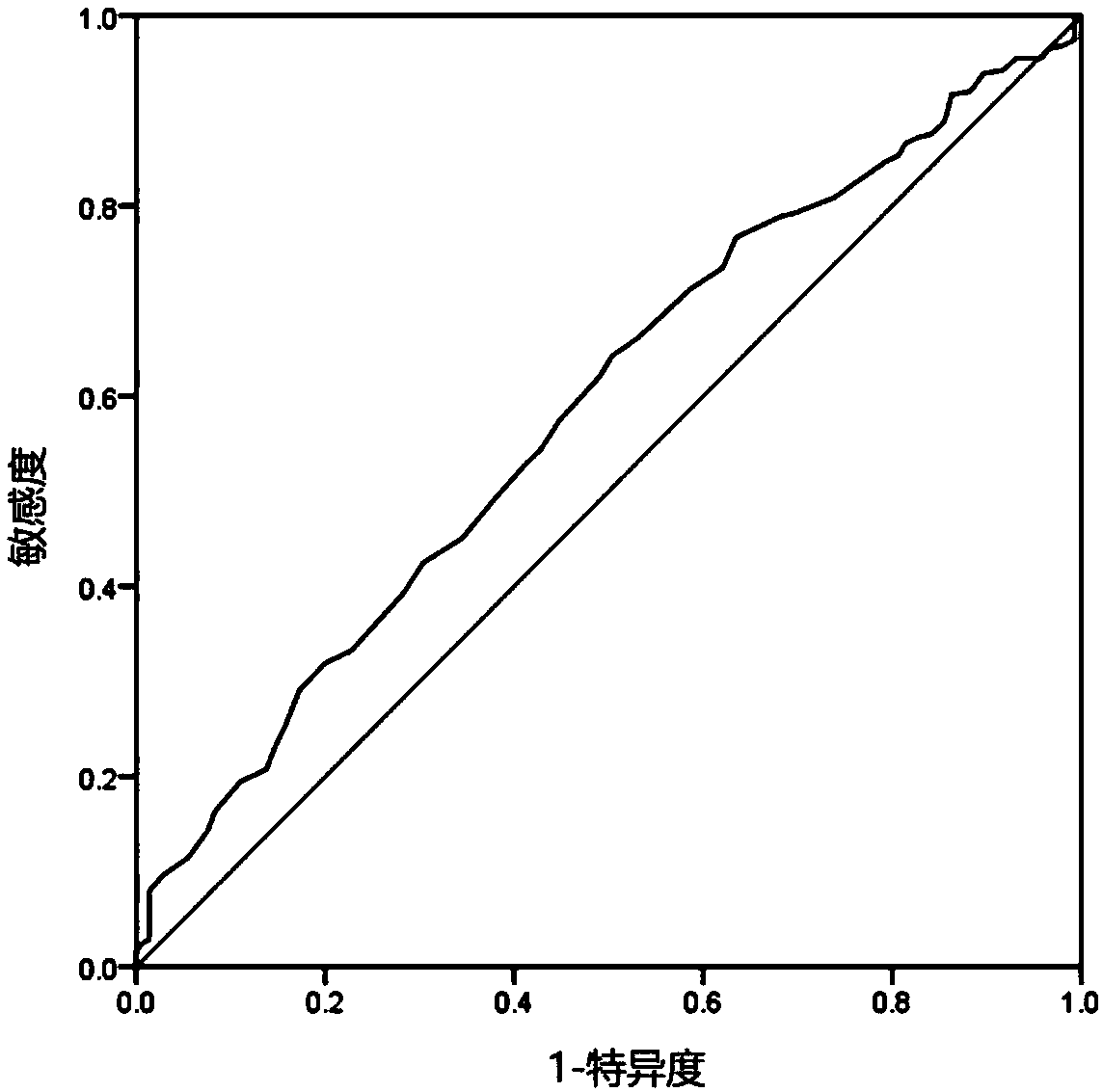
[0119] In the patient association study on possible adverse events after myocardial infarction, it was shown that the risk of adverse events after myocardial infarction was detected only by the thyroid-stimulating hormone level reagent of the present invention, without using the definition tools of blood pressure detection tools and medical history inquiry tools , when the detection value of thyroid-stimulating hormone level is 10.0IU / L, the correlation between the detected value and the risk of adverse events after myocardial infarction increased by 3.57 times. Obtain the receiver operating charac...
Embodiment 2
[0120] Example 2 Combined detection of thyroid-stimulating hormone and free triiodothyronine adverse events after myocardial infarction
[0121] The method for measuring the thyroid-stimulating hormone level by the patient's thyroid-stimulating hormone reagent is the same as that in Example 1. At the same time, 2ml of serum was extracted, and the free triiodothyronine concentration of the sample was measured according to the aforementioned free triiodothyronine detection method of this patent.
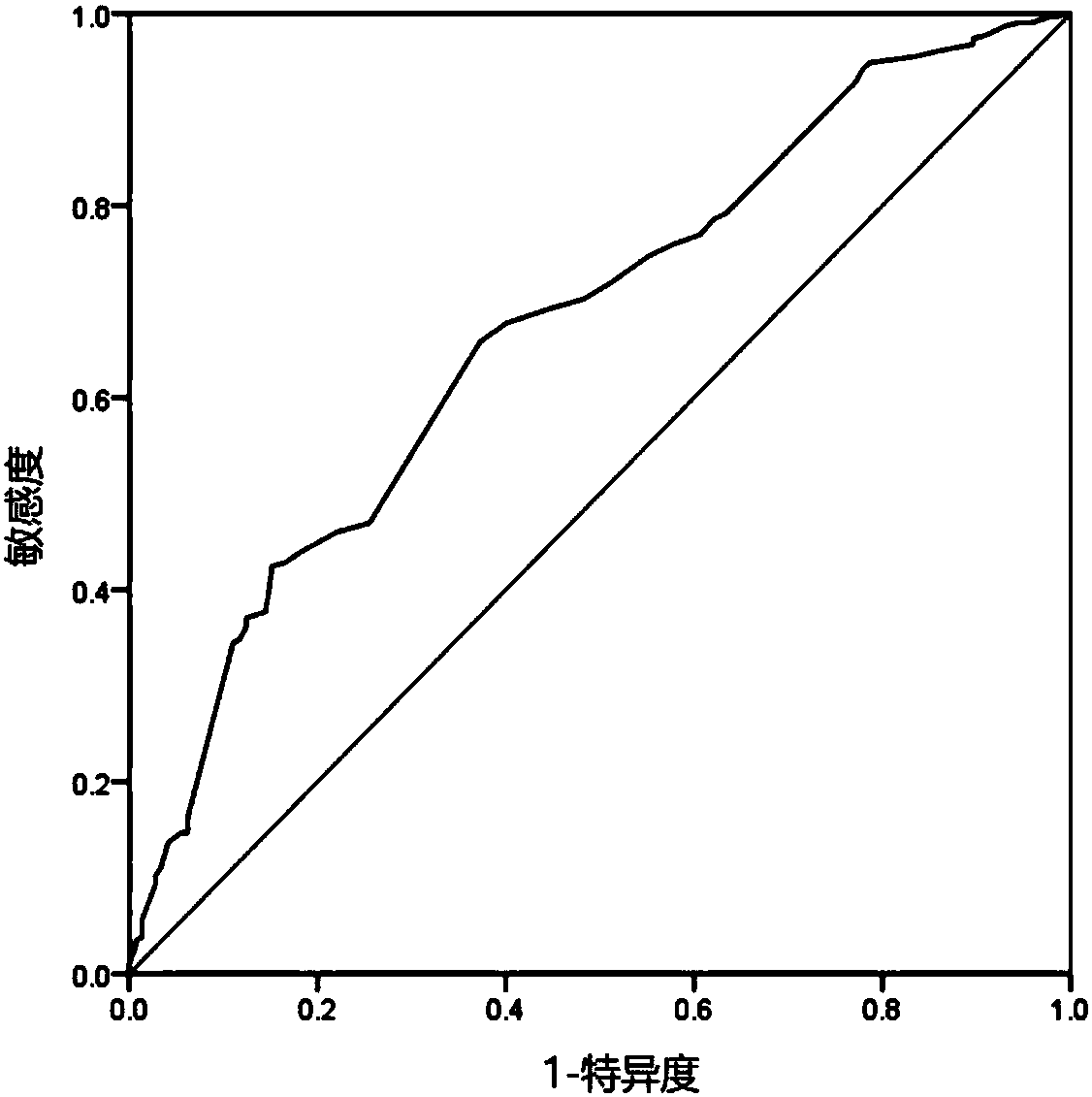
[0122] In the study on the association of patients with possible adverse events after myocardial infarction, it was shown that only through the combination reagent for detection of thyroid-stimulating hormone level and free triiodothyronine of the present invention, without using the definitions of blood pressure detection tools and medical history inquiry tools The tool detects the risk of adverse events after myocardial infarction. When the detection value of thyroid-stimulating horm...
Embodiment 3
[0123] Example 3 Thyroid-stimulating hormone, free triiodothyronine detection reagent and diabetes history detection tool combined detection of risk of adverse events after myocardial infarction
[0124] The method for measuring thyroid-stimulating hormone level and free triiodothyronine with the combined reagent of thyroid-stimulating hormone and leukocytes in patients is the same as in Example 1, and the history of diabetes is confirmed: those who have been clearly diagnosed with diabetes or received diabetes drug treatment; fasting blood glucose after admission ≥ 7.0mmol / L (126mg / dl) or any blood sugar ≥ 11.1mmol / L (200mg / dl).
[0125] In the study on the correlation of patients with possible adverse events after myocardial infarction, it was shown that the detection of thyroid-stimulating hormone levels, free triiodothyronine combined reagents and diabetes history detection tools of the present invention, without using blood pressure detection tools, has a positive effect ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com