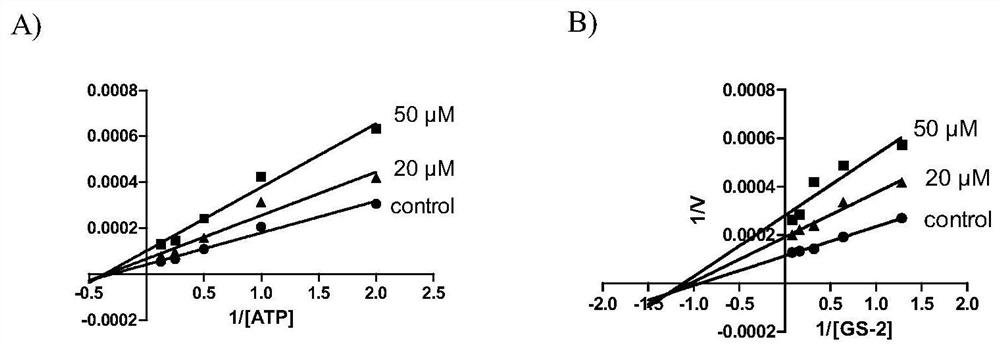
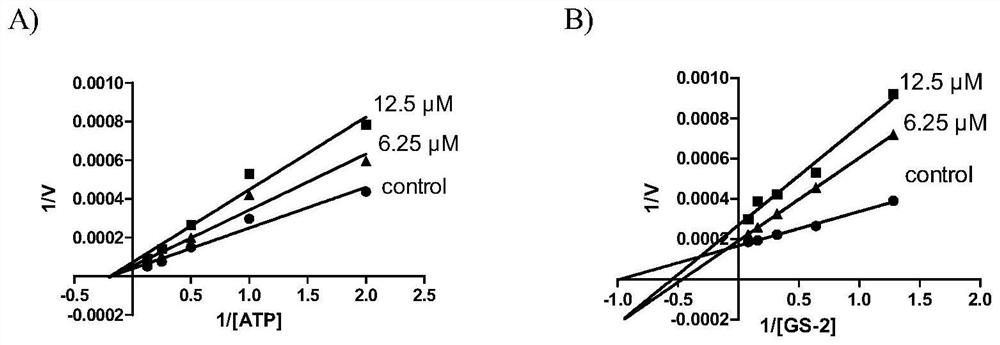
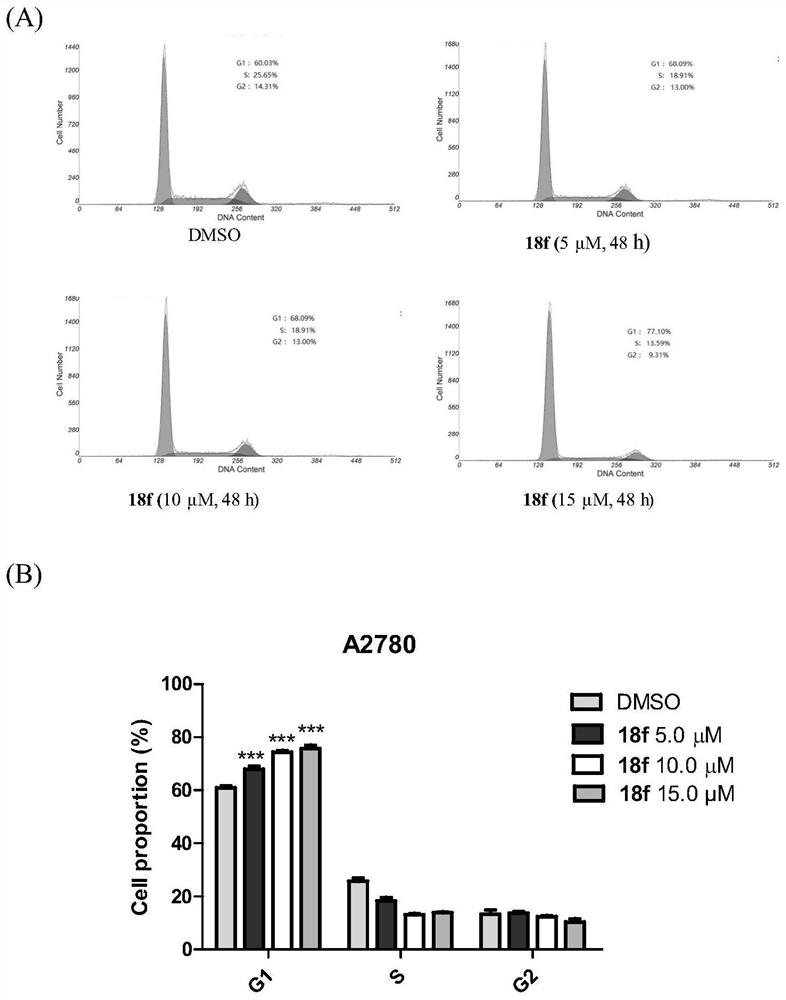
Benzothiazinone compound and its preparation method and pharmaceutical use
A benzothiazinone and compound technology, applied in the field of medicinal chemistry, can solve the problems of selectivity and specificity that cannot be ignored, and the effect of ovarian cancer is small.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Preparation of 4-ethyl-N-(1-nonanoylpiperidin-4-yl)-3-oxo-3,4-dihydro-(2H)benzo[1,4]thiazine-2-carboxamide ( 17a)
[0031] Preparation of 4-ethyl-(2H)1,4-benzothiazin-3(4H)-one (3)
[0032]
[0033] Into a 100 mL round bottom flask was added (2H)1,4-benzothiazin-3(4H)-one (2.5 g, 0.015 mol), sodium hydroxide powder (0.7 g, 0.016 mol), N,N-di Methylformamide (50mL) and ethyl bromide (1.5mL, 0.019mol) were reacted at room temperature, and the reaction was terminated by the disappearance of the raw material point as detected by TLC. Extracted with dichloromethane, combined organic layers, washed successively with 1N aqueous hydrochloric acid solution, saturated aqueous sodium bicarbonate solution, and saturated brine, dried over anhydrous sodium sulfate, concentrated under reduced pressure to remove solvent, and obtained 3.3 g of light yellow oil, which was directly used in Next reaction. Preparation of 2-methoxyformyl-4-ethyl-(2H)1,4-benzothiazin-3(4H)-one (5)
[0...
Embodiment 2
[0049] Preparation of N-(1-decanoylpiperidin-4-yl)-4-ethyl-3-oxo-3,4-dihydro-(2H)benzo[1,4]thiazine-2-carboxamide ( 17b)
[0050]
[0051] The preparation method was the same as that of 17a, except capric acid was used instead of nonanoic acid, the reaction solvent was N,N-dimethylformamide (DMF), the reaction temperature was 25°C, and the catalyst was TBTU / DIPEA to obtain a white flaky solid with a yield of 46%. 1 H NMR (400MHz, DMSO-d6) δ8.12 (d, J = 7.6Hz, 1H), 7.39 (d, J = 7.6Hz, 1H), 7.30-7.29 (m, 2H), 7.05-7.04 (m, 1H),4.25(s,1H),4.16-3.90(m,3H),3.76-3.65(m,2H),3.10-3.00(m,1H),2.75-2.67(m,1H),2.26-2.25( m, 2H), 1.76-1.24 (m, 18H), 1.14 (t, J=6.8Hz, 3H), 0.85 (t, J=6.6Hz, 3H).
Embodiment 3
[0053] Preparation of 4-ethyl-3-oxo-N-(1-undecanoylpiperidin-4-yl)-3,4-dihydro-(2H)benzo[1,4]thiazine-2-carboxamide (17c)
[0054]
[0055] The preparation method is the same as 17a, using undecanoic acid instead of pelargonic acid, white flaky solid, yield 63%. 1 H NMR (400MHz, DMSO-d6) δ8.12 (d, J = 7.2Hz, 1H), 7.39 (d, J = 7.2Hz, 1H), 7.30-7.29 (m, 2H), 7.06-7.04 (m, 1H),4.25(s,1H),4.16-3.90(m,3H),3.76-3.65(m,2H),3.09-3.00(m,1H),2.75-2.67(m,1H),2.26-2.25( m, 2H), 1.76-1.24 (m, 20H), 1.14 (t, J = 7.0Hz, 3H), 0.85 (t, J = 6.4Hz, 3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com