Specific antibody with CD20 as target, CAR-NK cell and preparation and application of CAR-NK cell
A specificity and antibody technology, applied in the field of biomedicine, can solve the problems of high production cost, inability to reinfuse the allogeneic body, unfavorable large-scale application, etc., and achieve the effect of stable traits
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Example 1 Preparation of lentiviral expression vector
[0060]Gene synthesis ScFV (Anti CD20)-CD8-4-1BB-CD3ζ fusion gene sequence (the amino acid sequence is shown in SEQ ID NO: 11, and the gene sequence is shown in SEQ ID NO: 12). Through enzyme digestion, it was transformed and connected to the PRRSLIN vector, and the upstream of the gene was the EP-1α promoter. Transform the Stbl3 E. coli strain with the vector, screen with ampicillin, obtain positive clones, extract the plasmids, identify the clones by restriction enzyme digestion, and obtain the PRRLSIN-SCFV (Anti CD20)-CD8-4-1BB-CD3ζ lentiviral transfection vector (such as figure 1 shown).
Embodiment 2
[0061] The preparation of embodiment 2 lentiviruses
[0062] (1) 24 hours before transfection, use about 8×10 per dish 6 293T cells were seeded into 15cm culture dishes. Make sure that the cells are at about 80% confluence and evenly distributed in the culture dish during transfection.
[0063] (2) Prepare solution A and solution B
[0064] Solution A: 6.25ml 2×HEPES buffer (the amount packed in 5 large dishes, the effect is the best).
[0065] Solution B: Add the mixture of the following plasmids: 112.5 μg PRRLSIN-SCFV(2-27)-CD8-4-1BB-CD3ζ (target plasma); 39.5 μg pMD2.G (VSV-G envelope); 73 μg pCMVR8.74 (gag , pol, tat, rev); 625 μl of 2M calcium ion solution. Total volume of solution B: 6.25ml.
[0066] Mix solution B thoroughly, and while vortexing solution A gently, add solution B drop by drop and let stand for 5-15 minutes. Gently vortex the above mixed solution of A and B, add dropwise to the culture dish containing 293T cells, gently shake the culture dish back a...
Embodiment 3
[0067] Example 3 Preparation of CD20 CAR NK-92 cells
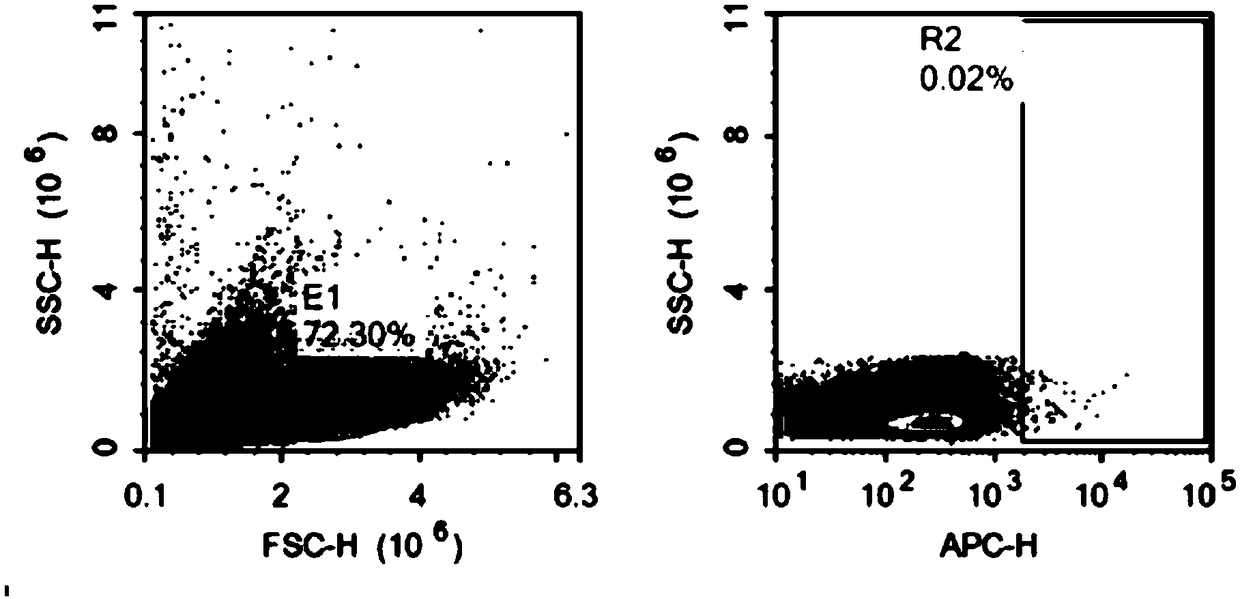
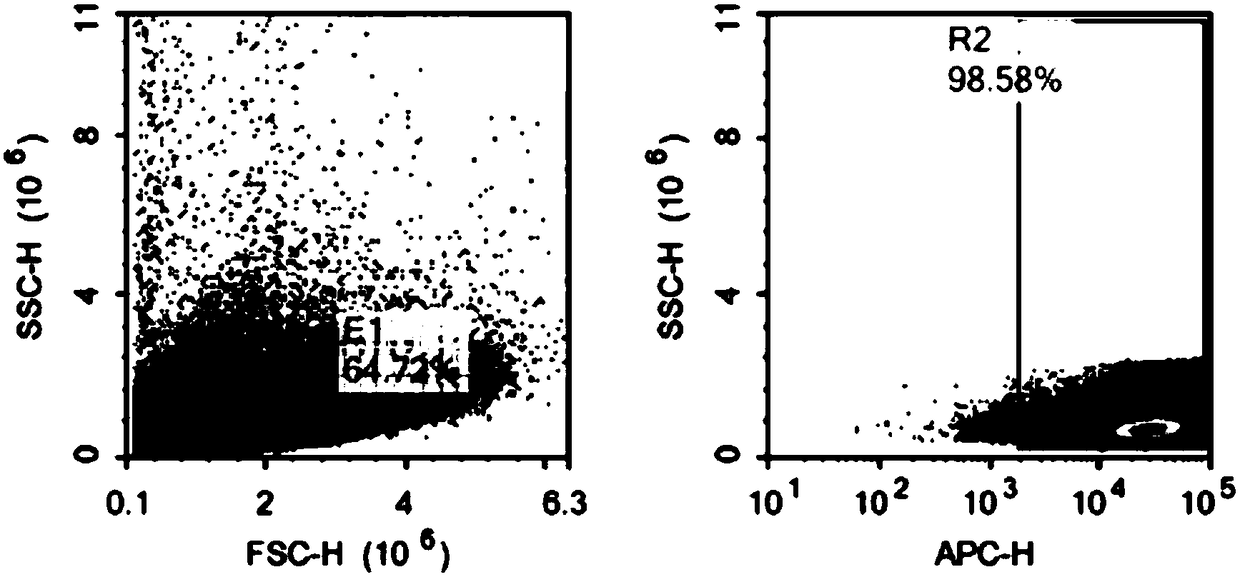
[0068] Adjust the NK-92 cell density to 2-3 x 10 5 / ml, add the virus vector (prepared in Example 2) according to the ratio of volume ratio (V / V) virus vector: cell culture medium=1:5-10, and add polybrene 8 μg / ml at the same time. After 4 h, add an equal amount of fresh complete medium to adjust the cell density to 1×10 5 / ml to continue the culture. The next day, all the cells were centrifuged, fresh medium was added, and the culture was continued. Replenish fluid every 1-2 days to maintain cell density at 2-3×10 5 / ml. After 72 hours, CAR antibody staining was performed, and CD20 CAR NK-92 positive cells were sorted by flow cytometry and expanded for culture. Observe the color change of the medium, cell density, and cell shape every day and make corresponding records.
[0069] The positive rate of CAR NK-92 cells was detected by flow cytometry, and the results of flow cytometry were as follows: Figure 2a and F...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com