a co 2 Cycloaddition cocatalysts and their applications in cycloaddition reactions
A co-catalyst and cycloaddition technology, applied in physical/chemical process catalysts, organic compound/hydride/coordination complex catalysts, 3/13 group organic compounds without C-metal bonds, etc., can solve epoxy The problem of low catalytic activity, poor selectivity, and difficulty in separating the catalyst from the product can be solved, so as to achieve the effect of increasing the yield, good selectivity, and good catalytic activity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0083] The preparation of embodiment 1 sample GdL
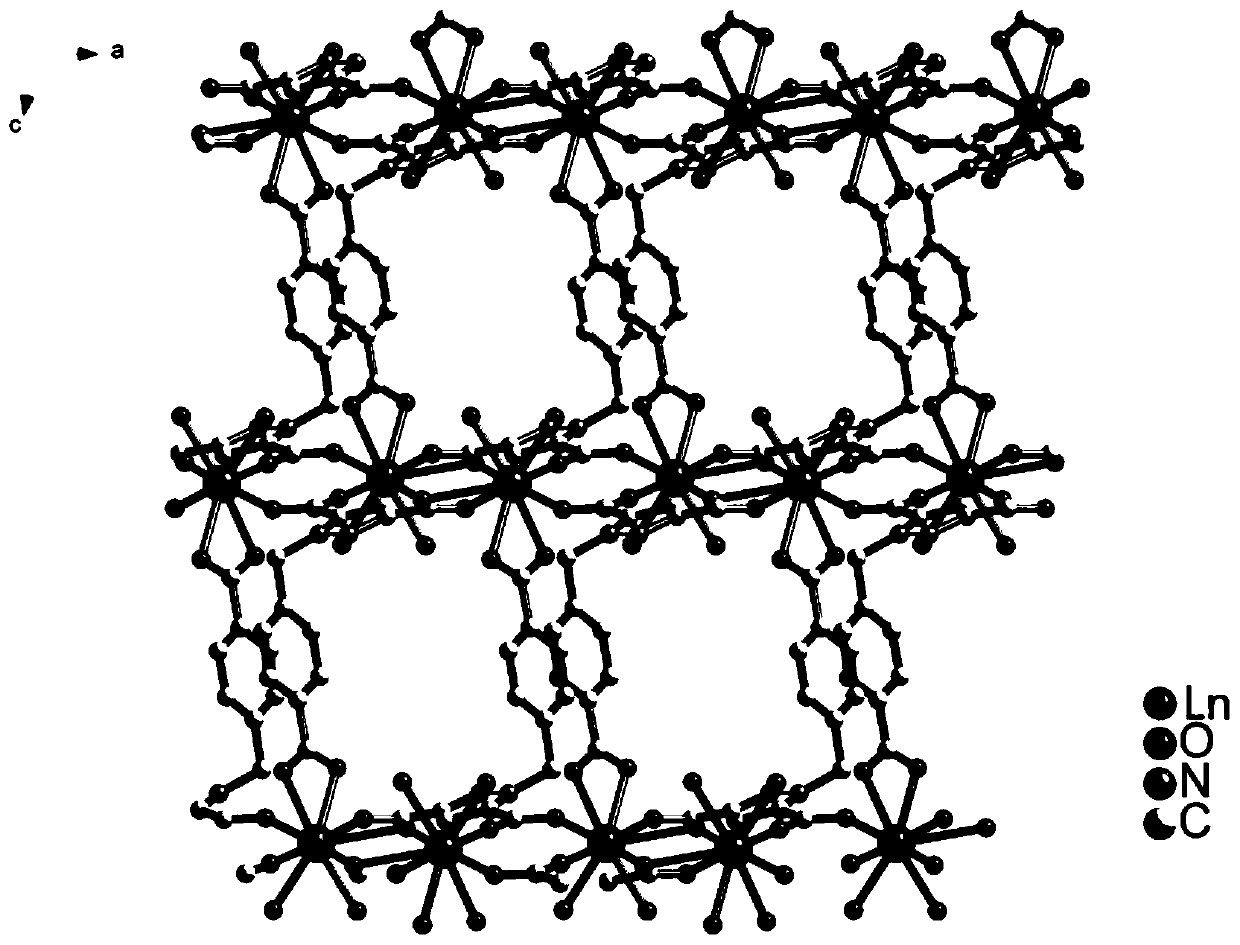
[0084] The organic ligand 1-(4-benzoic acid)-1 hydrogen-pyrazole-3,5-dicarboxylic acid and gadolinium nitrate were dissolved in CH at a molar ratio of 1:1 3 Mixed solvent of CN and water (volume ratio CH 3 CN: water=1:1) to obtain a solution; the concentration of gadolinium nitrate in the solution is 0.1mol / L. Put the above solution into a closed hydrothermal kettle, keep it at 130°C for 5 days, centrifuge the obtained solid, and dry it in vacuum to obtain the rare earth organic framework crystal material, which is designated as sample GdL.
Embodiment 2
[0085] Preparation of Example 2 Sample EuL
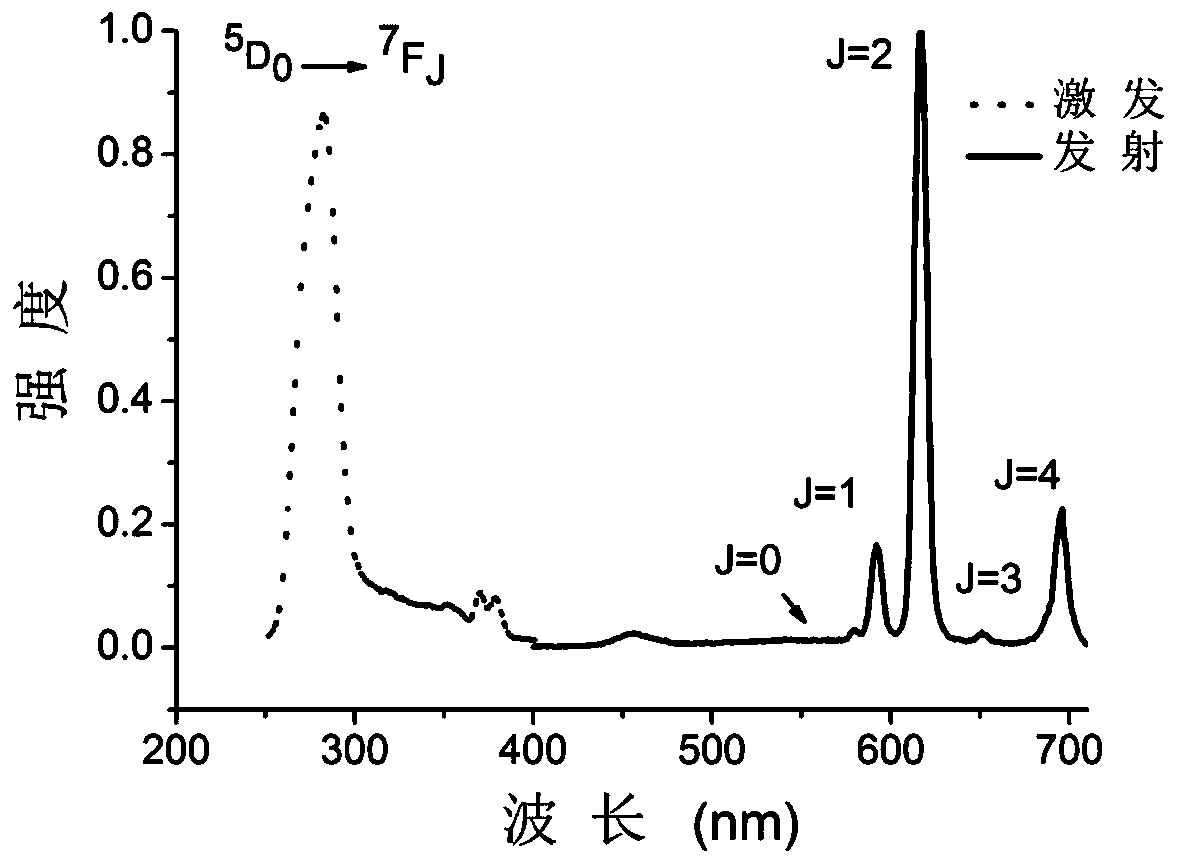
[0086]The organic ligand 1-(4-benzoic acid)-1 hydrogen-pyrazole-3,5-dicarboxylic acid and europium nitrate were dissolved in CH at a molar ratio of 1:1 3 Mixed solvent of CN and water (volume ratio CH 3 CN: water=1:1) to obtain a solution; the concentration of gadolinium nitrate in the solution is 0.5mol / L. Put the above solution into a closed hydrothermal kettle, keep it at 150°C for 1 day, centrifuge the obtained solid, and dry it in vacuum to obtain the rare earth organic framework crystal material, which is recorded as sample EuL.
Embodiment 3
[0087] The preparation of embodiment 3 sample TbL
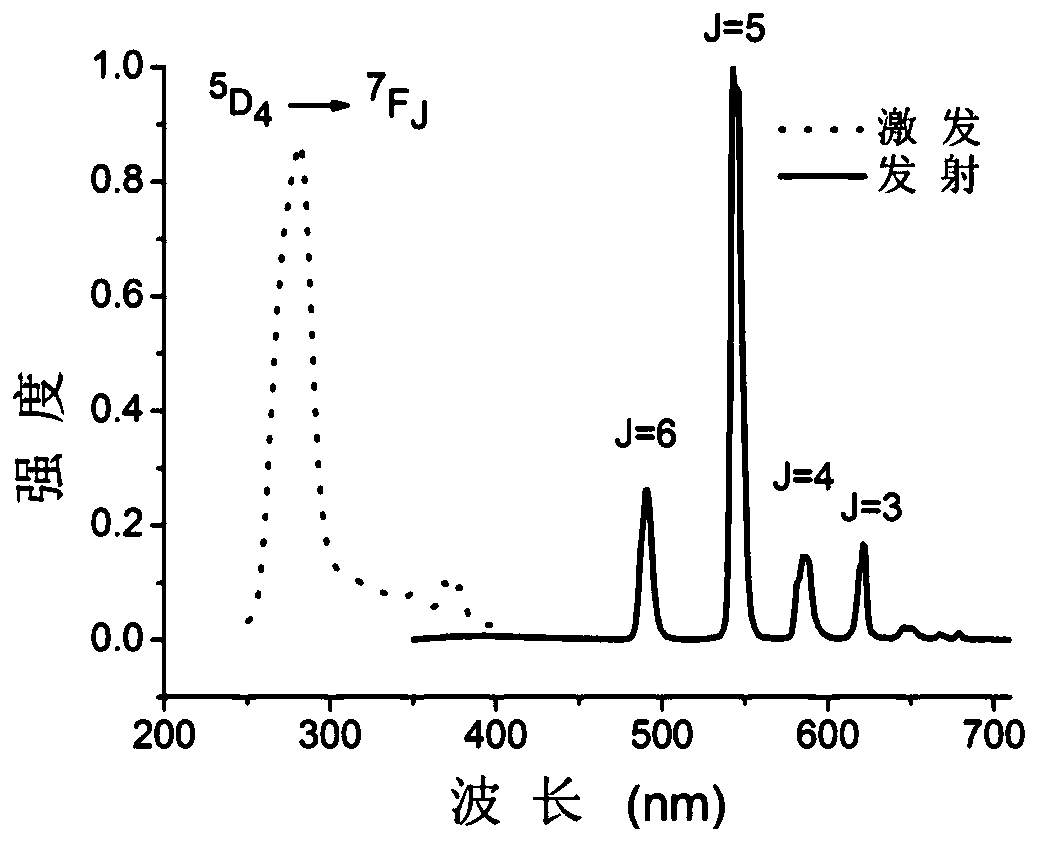
[0088] The organic ligand 1-(4-benzoic acid)-1 hydrogen-pyrazole-3,5-dicarboxylic acid and terbium nitrate were dissolved in CH at a molar ratio of 1:1 3 Mixed solvent of CN and water (volume ratio CH 3 CN: water=1:1) to obtain a solution; the concentration of gadolinium nitrate in the solution is 0.3mol / L. Put the above solution into a closed hydrothermal kettle, keep it at 140°C for 3 days, centrifuge the obtained solid, and dry it in vacuum to obtain the rare earth organic framework crystal material, which is designated as sample TbL.
PUM
| Property | Measurement | Unit |
|---|---|---|
| decomposition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com