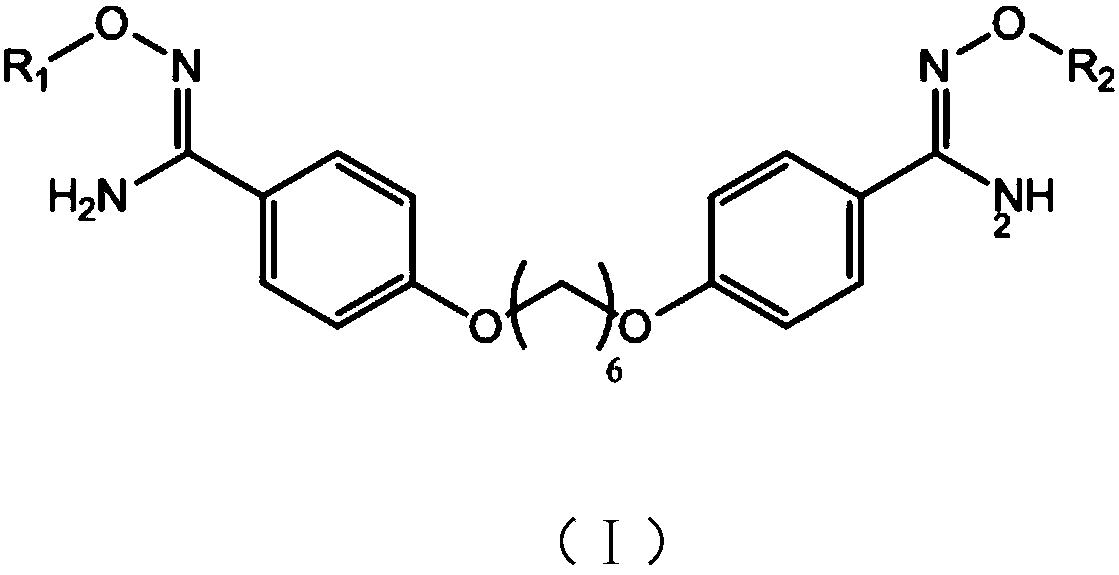
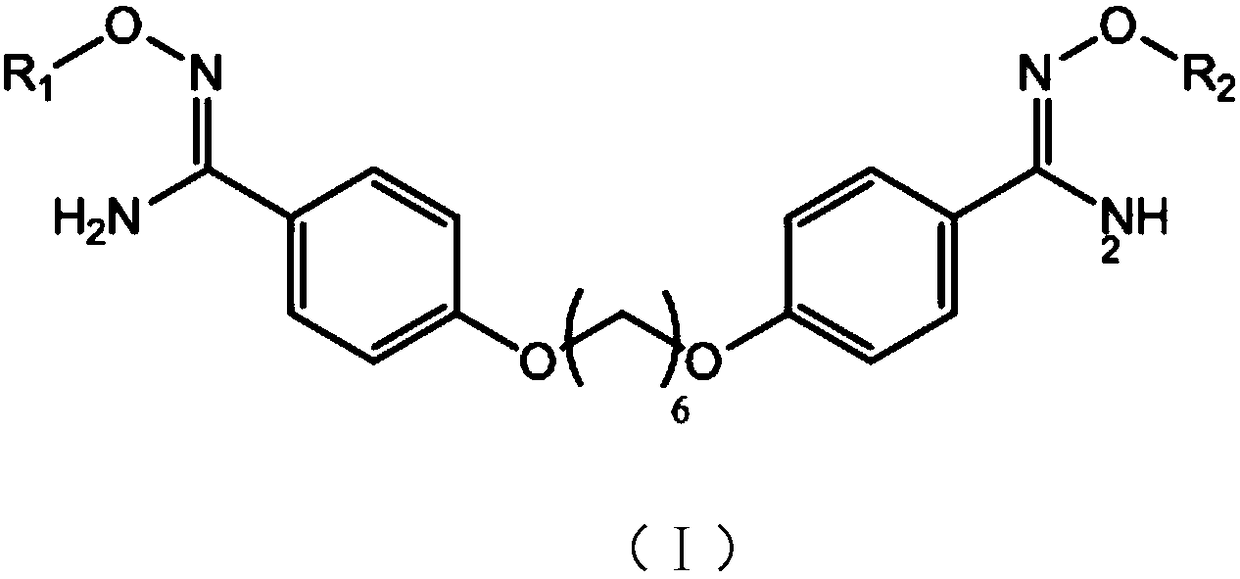
New synthesizing method for 1,6-(paramidyl phenyl)dihexyl ether
The technology of an amidino phenyl group and a synthesis method, which is applied in 1 field, can solve the problems of small industrial value, pollution, low yield of intermediate B, etc., and achieves the effects of reducing production cost, safe and easy-to-obtain raw materials, and improving total yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] A kind of embodiment of the new synthetic method of 1,6-(p-amidinophenyl) hexylene diether of the present invention, comprises the following steps:
[0044] (1) Synthesis of 1,6-(p-cyanophenyl) hexamethylene diether:
[0045] In a 1L four-neck flask equipped with a stirring, thermometer, dropping funnel and reflux condenser, add 500ml of ethanol and 57.16g (0.840mol; 1.5eq) of sodium ethylate in sequence, stir to dissolve, then add 100g (0.840 mol; 1.5eq) p-cyanophenol, then heated up to 60°C and refluxed for 1 hour, then slowly added 136.6g (0.560mol; 1eq) 1,6-dibromohexane dropwise at this temperature, and maintained the temperature after dropping Sampling was carried out after 4 hours of reaction, the reaction was completed by liquid phase monitoring, the temperature was lowered to 20°C, and after stirring for 1 hour, it was filtered to obtain a solid wet product. After beating with 300 ml of water, it was filtered and dried to obtain 121.0 g of a white solid, which ...
Embodiment 2
[0054] A kind of embodiment of the new synthetic method of 1,6-(p-amidinophenyl) hexylene diether of the present invention, comprises the following steps:
[0055] (1) Synthesis of 1,6-(p-cyanophenyl) hexamethylene diether:
[0056] In a 1L four-neck flask equipped with stirring, a thermometer, a dropping funnel, and a reflux condenser, add 500ml of ethanol and 57.16g (0.840mol; 2.2eq) of sodium ethoxide in sequence, stir to dissolve it completely, cool to room temperature, and divide Add 100g (0.840mol; 2.2eq) of p-cyanophenol in batches, then raise the temperature to 80°C and react for 1h, then add 93.2g (0.382mol; 1eq) of 1,6-dibromohexane dropwise under the control of the temperature, and keep React at this temperature for 1 hour and take samples. The liquid phase monitors that the reaction is complete. Cool down to 25°C, stir for 1 hour, and filter it to obtain a solid wet product. After beating with 300ml of water, filter and dry to obtain 116.8g of a white solid, which ...
Embodiment 3
[0066] A kind of embodiment of the new synthetic method of 1,6-(p-amidinophenyl) hexylene diether of the present invention, comprises the following steps:
[0067] (1) Synthesis of 1,6-(p-cyanophenyl)hexylene diether: In a 1L four-neck flask equipped with a stirring, thermometer, dropping funnel and reflux condenser, add 500ml of ethanol and 57.2g (0.840 mol; 2eq) sodium ethylate, stir to dissolve it completely, add 100g (0.840mol; 2eq) of p-cyanophenol in batches, then raise the temperature to 65°C and reflux for 1h, then add 102.5g (0.42mol; 1eq) dropwise under reflux 1,6-dibromohexane, after dripping, keep the reaction at the temperature for 10 hours to take samples, monitor the reaction in liquid phase, cool down to 22°C, stir for 1 hour, filter it to obtain a solid wet product, beat with 300ml of water, filter and dry, 125.2 g of white solid was obtained, which was 1,6-(p-cyanophenyl)hexamethylene ether;
[0068] The yield of 1,6-(p-cyanophenyl)hexamethylene diether in t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com