All-solid-state composite polymer electrolyte and preparation method thereof
A composite polymer and electrolyte technology, which is applied in the direction of solid electrolyte, non-aqueous electrolyte, non-aqueous electrolyte battery, etc., can solve the problems of battery temperature rise, combustion or explosion, and difficulty in molding, and achieve simple preparation process and high conductivity , the effect of high production efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] An all-solid composite polymeric solid electrolyte, comprising:
[0025] PEO, 62.41wt%;
[0026] Lithium salt LiN(SO 2 CF 3 ) 2 , 22.59wt%;
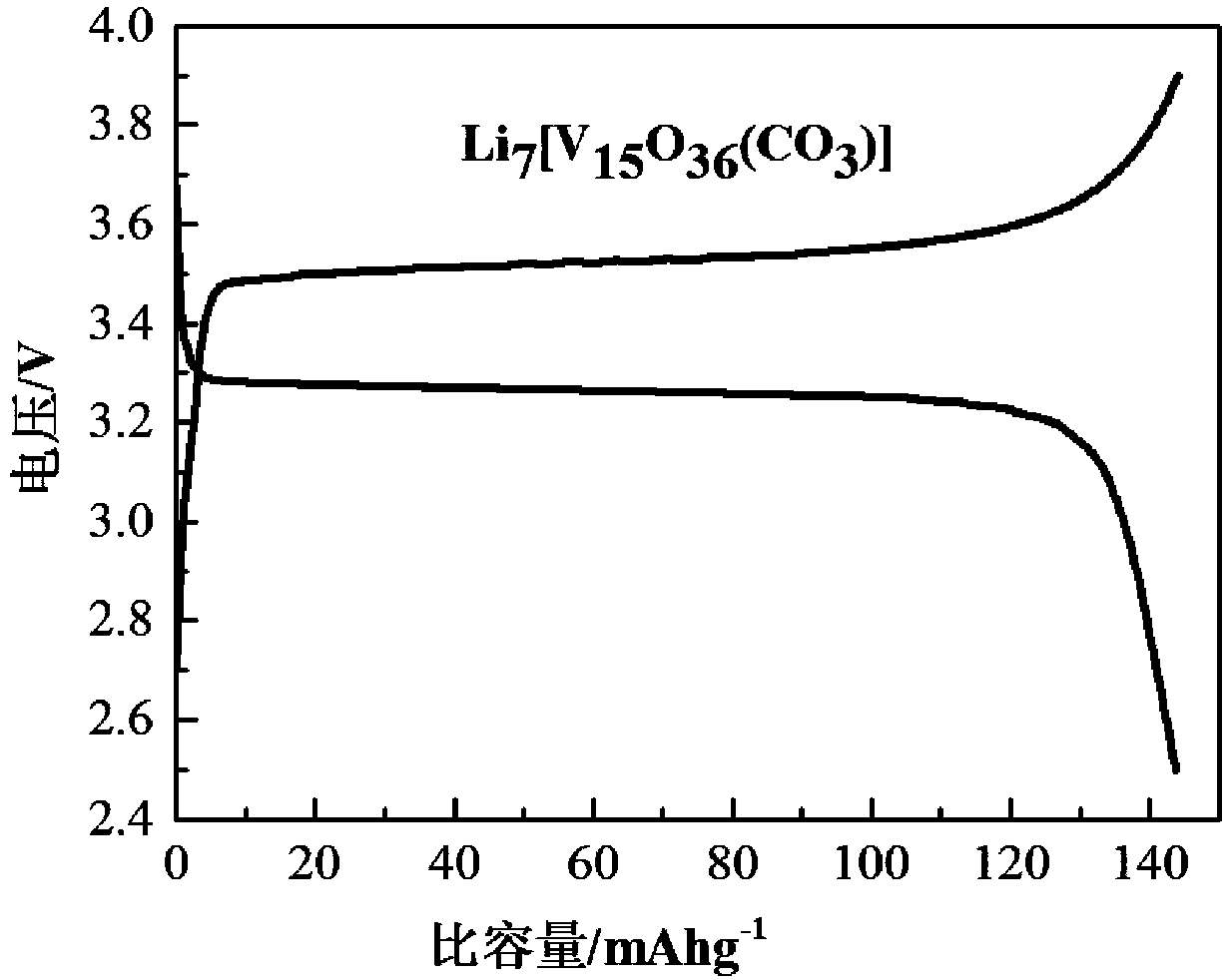
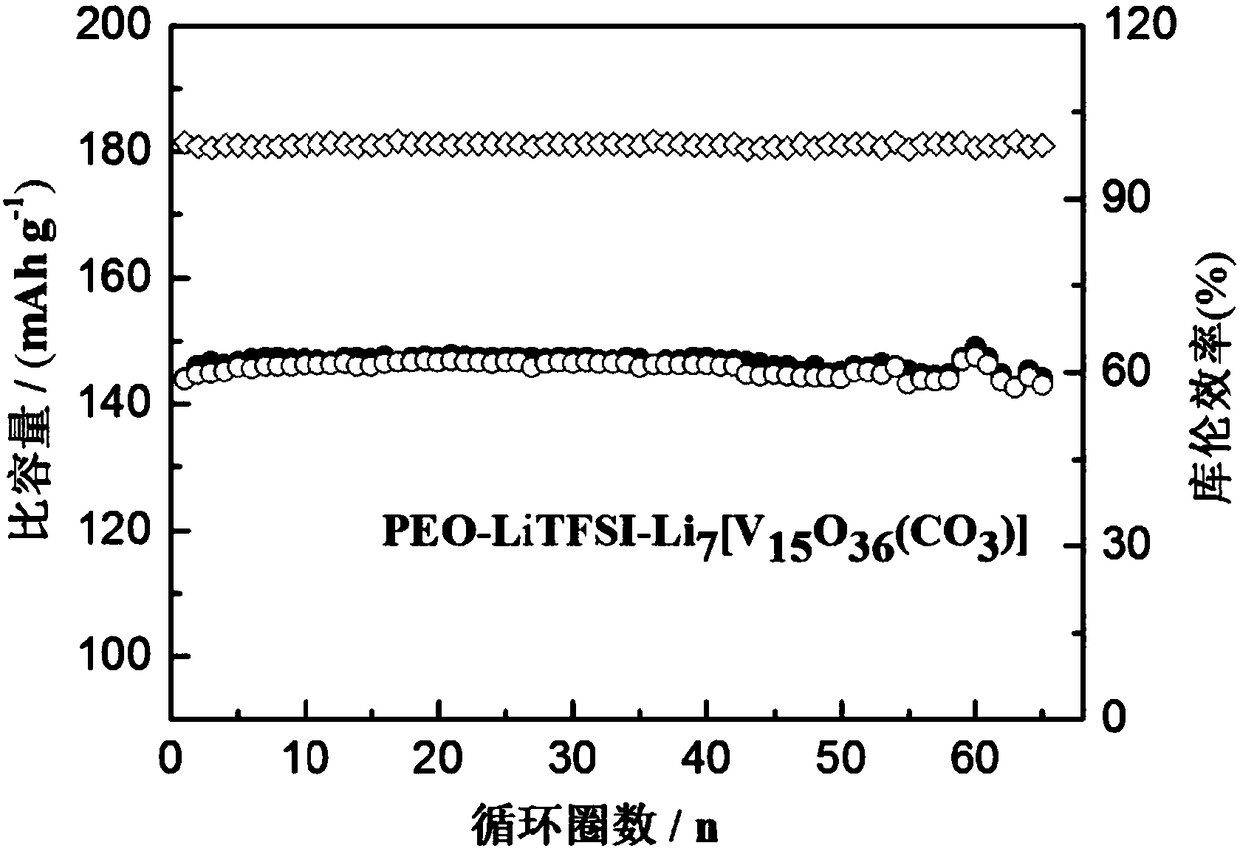
[0027] Li 7 [V 15 o 36 (CO 3 )], 15.00 wt%.
[0028] The steps of the preparation method of the above-mentioned solid composite polymer electrolyte membrane are as follows:
[0029] (1) Weigh 0.6 g of PEO powder, add it into 25 mL of acetonitrile solution, and stir for 24 hours to obtain a uniform PEO-acetonitrile solution.
[0030] (2) Lithium salt LiN(sO 2 CF 3 ) 2 0.2172g, Li 7 [V 15 o 36 (CO 3 )] 0.1442g was added in 15mL acetonitrile solution, stirred for 24h to obtain a uniform LiN(SO 2 CF 3 ) 2 -Li 7 [V 15 o 36 (CO 3 )]-acetonitrile solution.
[0031] (3) the PEO-acetonitrile solution obtained in step (1) and the LiN(SO4) obtained in step (2) 2 CF 3 ) 2 -Li 7 [V 15 o 36 (CO 3 )]-acetonitrile solution was mixed and stirred for 12h. In a glove box filled with argon, pour the obtained mixed solu...
Embodiment 2
[0034] An all-solid composite polymeric solid electrolyte, comprising:
[0035] PEO, 67.88wt%;
[0036] Lithium salt LiN(SO 2 CF 3 ) 2 , 22.12wt%;
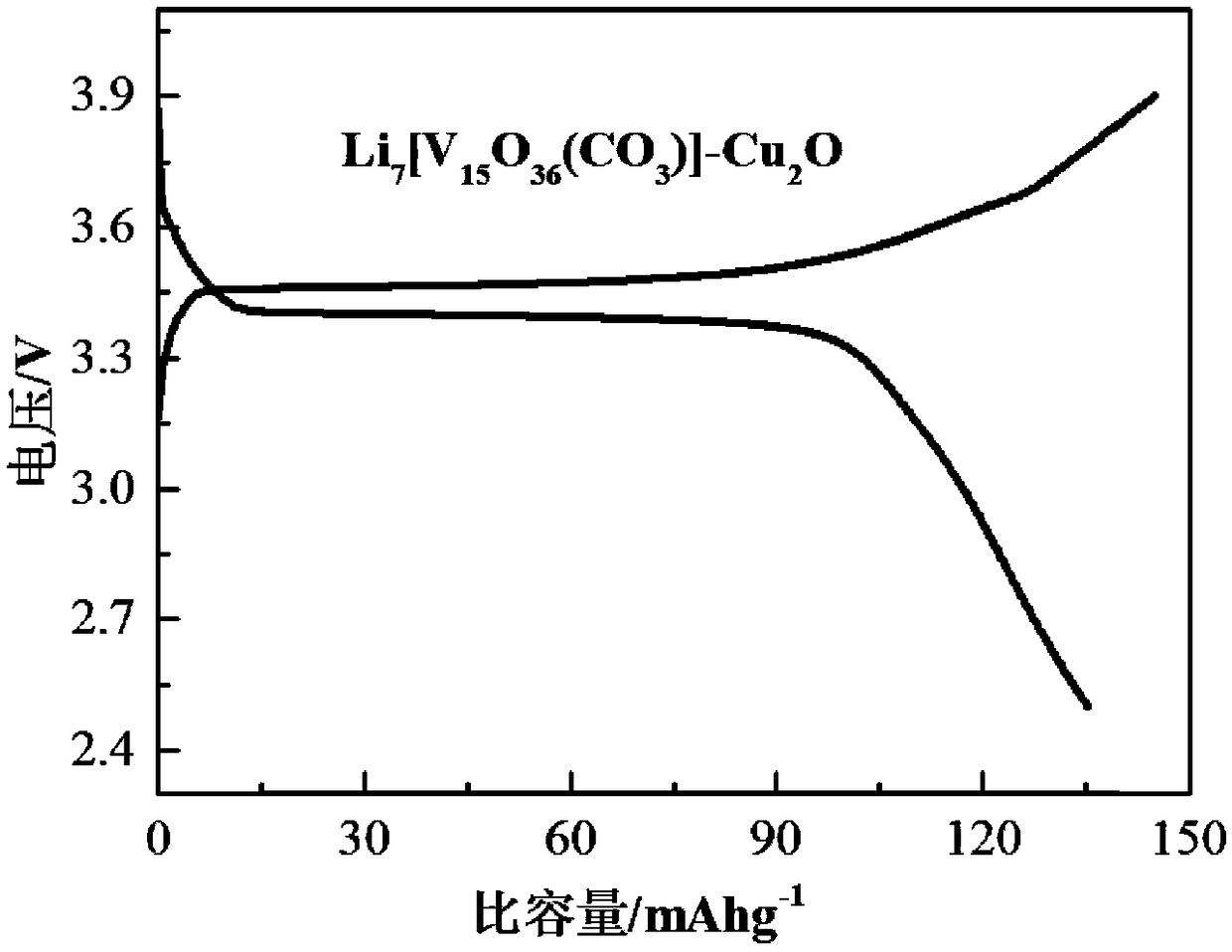
[0037] Li 7 [V 15 o 36 (CO 3 )], 5.00wt%;
[0038] Cu 2 O, 5.00 wt%.
[0039] The steps of the preparation method of the above-mentioned solid composite polymer electrolyte membrane are as follows:
[0040] (1) Weigh 0.6 g of PEO powder, add it into 25 mL of acetonitrile solution, and stir for 24 hours to obtain a uniform PEO-acetonitrile solution.
[0041] (2) Lithium salt LiN(SO 2 CF 3 ) 2 0.1955g, Li 7 [V 15 o 36 (CO 3 )] 0.04420g and Cu 2 O0.04420g was added in 15mL acetonitrile solution, stirred for 24h to obtain a uniform LiN(SO 2 CF 3 ) 2 -Li 7 [V 15 o 36 (CO 3 )]-Cu 2 O-acetonitrile solution.
[0042] (3) the PEO-acetonitrile solution obtained in step (1) and the LiN(SO4) obtained in step (2) 2 CF 3 ) 2 -Li 7 [V 15 o 36 (CO 3 )]-Cu 2 The O-acetonitrile solution was mixed and stirred for ...
Embodiment 3
[0045] An all-solid composite polymeric solid electrolyte, comprising:
[0046] PEO, 66.08wt%;
[0047] Lithium salt LiN(SO 2 CF 3 ) 2 , 23.92wt%;
[0048] Li 7 [V 15 o 36 (CO 3 )], 10.00wt%;
[0049] The steps of the preparation method of the above-mentioned solid composite polymer electrolyte membrane are as follows:
[0050] (1) Weigh 0.6 g of PEO powder, add it into 25 mL of acetonitrile solution, and stir for 24 hours to obtain a uniform PEO-acetonitrile solution.
[0051] (2) will Li 2 CO 3 Add 0.0813g into 10mL acetonitrile solution, stir for 2h to form Li 2 CO 3 - acetonitrile suspension.
[0052] (3) the PEO-acetonitrile solution obtained in step (1) and the Li obtained in step (2) 2 CO 3 -The acetonitrile suspension was mixed and stirred for 5h to obtain PEO-Li 2 CO 3 - acetonitrile solution.
[0053] (4) put V 2 o 5 0.1748g slowly adds the PEO-Li of step (3) gained several times 2 CO 3 -Acetonitrile solution, stirred for 5min.
[0054] (5) He...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com