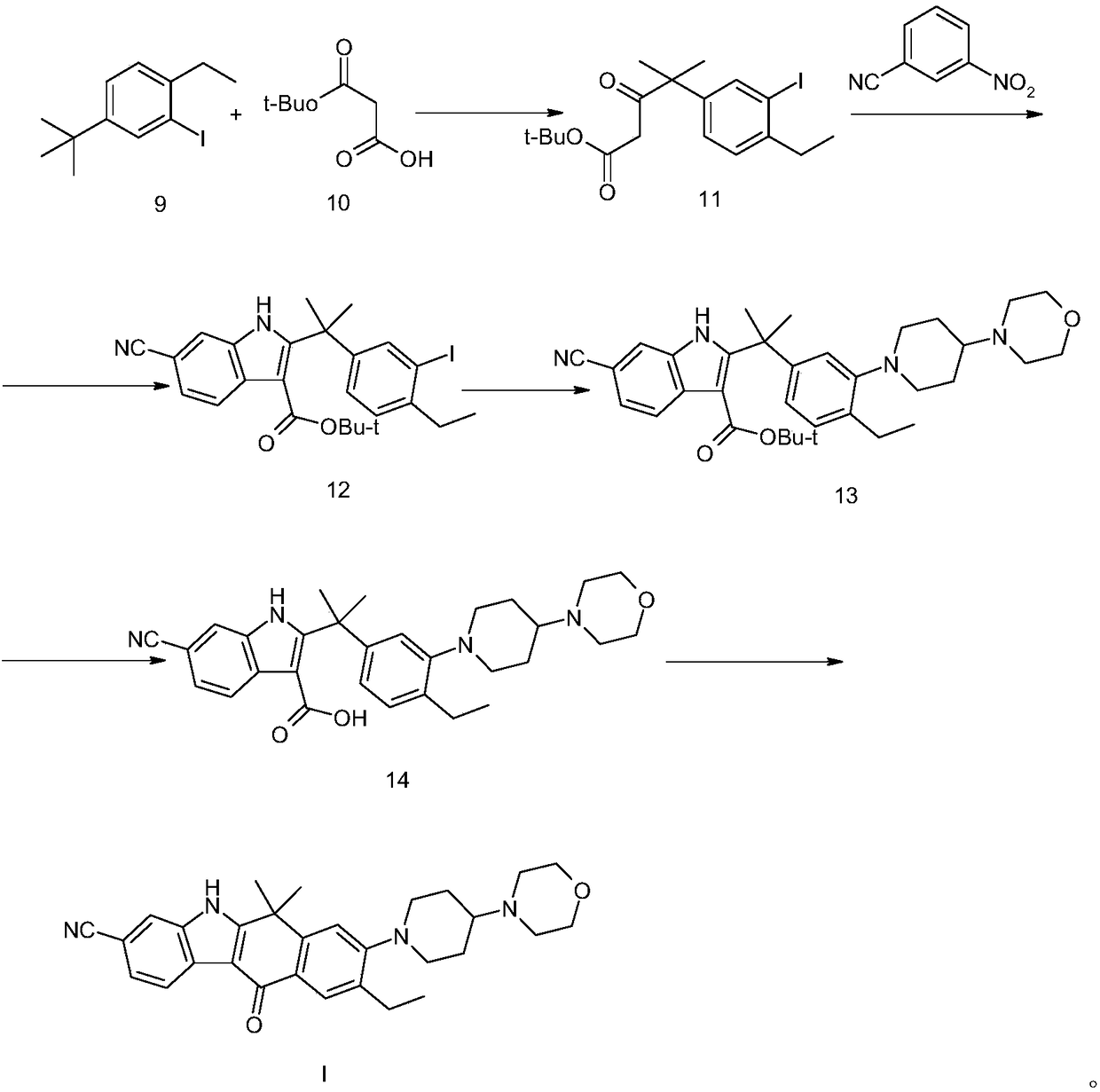
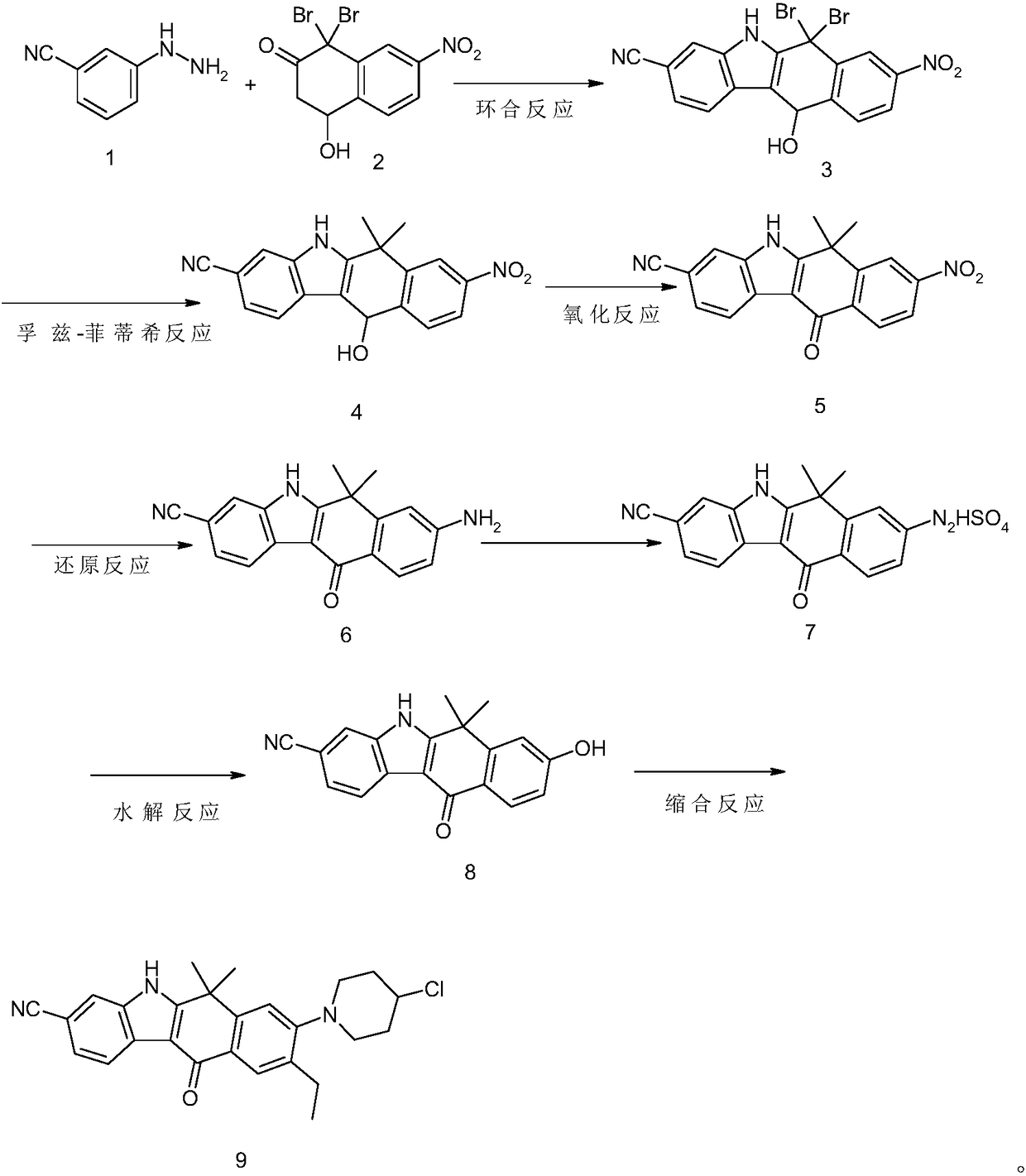
Method for preparing alectinib intermediate
A technology of intermediates and tinib, which is applied in the field of medicine, can solve the problems of difficult acquisition of reaction raw materials, many side reactions, and difficult purification, and achieve the effects of low cost, high total yield, and short synthesis steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] A preparation method of alritinib intermediate, comprising the following processing steps:
[0052] A. Cyclization reaction
[0053] Dissolve 90% hydrochloric acid (30ml) in water (60ml) to prepare a hydrochloric acid solution, add 3-cyanophenylhydrazine ① (22g, 2.4mol) and 6-nitro-4,4-dibromo-1-hydroxy- 1,2,3,4-tetrahydronaphthalene-3 ketone ② (25g, 2.78mol) mixture, after stirring, add reaction accelerator boron trifluoride (5.4g, 1.7mol) and organic solvent 1,2-dichloro Ethane (40ml), N, N-dimethylformamide (DMF) (40ml), heated in a water bath to 20°C, after 3 hours of reaction, continue to dropwise add hydrochloric acid solution (30ml) and oxidative dehydrogenation agent to the reaction solution Chlorobenzoquinone (3.9g, 1.5mol), fully stirred, heated to 60°C for ring closure reaction, cooled to 2°C in an ice-water bath after the reaction, removed the organic solvent, and recrystallized to obtain 6,6-dibromo- 8-nitro-11-hydroxy-6,11-dihydro-5H-benzo[b]carbazole-3-...
Embodiment 2
[0065] A preparation method of alritinib intermediate, comprising the following processing steps:
[0066] A. Cyclization reaction
[0067] Dissolve 90% hydrochloric acid (60ml) in water (120ml) to prepare a hydrochloric acid solution, add 3-cyanophenylhydrazine ① (60g, 5.8mol) and 6-nitro-4,4-dibromo-1-hydroxyl- 1,2,3,4-tetrahydronaphthalene-3 ketone ② (57g, 4.6mol) mixture, after stirring, add reaction accelerator boron trifluoride (11.5g, 2.6mol) and organic solvent 1,2-dichloro Ethane (80ml), N,N-dimethylformamide (DMF) (80ml), heated to 25°C in a water bath, after 4.5 hours of reaction, continue to dropwise add hydrochloric acid solution (100ml) and oxidative dehydrogenation agent to the reaction solution Chlorobenzoquinone (5.9g, 2.7mol), fully stirred, heated to 64°C for ring closure reaction, cooled to 5°C in an ice-water bath after the reaction, removed the organic solvent, and recrystallized to obtain 6,6-dibromo- 8-nitro-11-hydroxy-6,11-dihydro-5H-benzo[b]carbazol...
PUM
| Property | Measurement | Unit |
|---|---|---|
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap