Drug delivery device
一种递送装置、药物的技术,应用在药物输送、药物组合、药物的器械等方向,能够解决无法调节给药量、低血糖、症状恶化等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0106]The gelling agent (that is, the skeleton structure) uses N-isopropylmethacrylamide (NIPMAAm) and phenylboronic acid monomer (AmECFPBA), and the crosslinking agent uses N,N'-methylenebisacrylamide (MBAAm) , the polymerization initiator uses 2,2'-azobisisobutyronitrile, the above four are mixed at a feed molar ratio of 91.5 / 7.5 / 1 / 0.1, and radical polymerization is carried out in a capillary with a diameter of 1 mm to prepare a gel.
[0107] [chemical 4]
[0108]
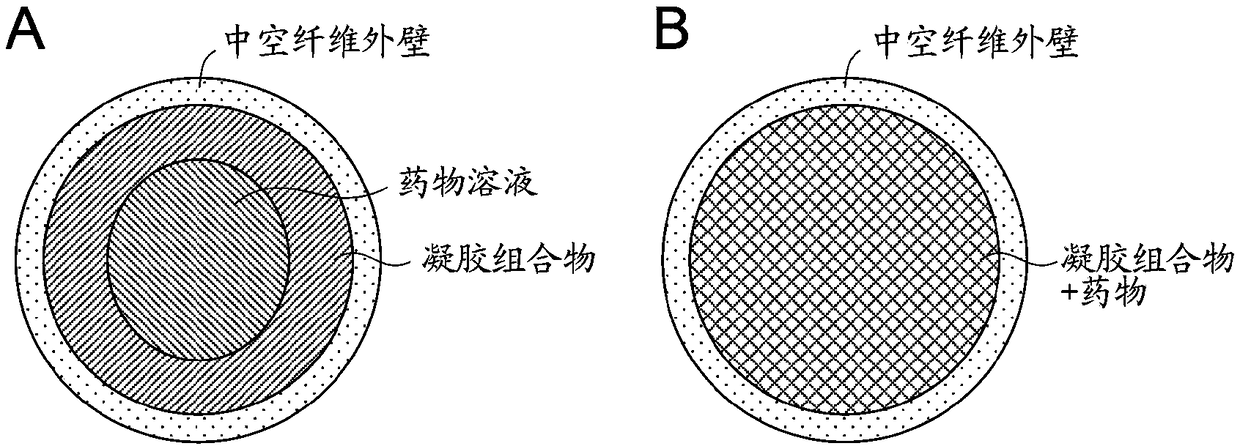
[0109] The obtained gel was swelled in a human insulin preparation (Humulin R injection, manufactured by Eli Lilly) solution or PBS at room temperature, and then soaked in 0.1M hydrochloric acid aqueous solution at 37° C. for 1 hour to encapsulate insulin.
[0110] Under physiological conditions (pH 7.4, 37°C), the gel can undergo a phase transition with the normoglycemic level (1g / L) as the threshold, and it was confirmed that the release of insulin is controlled using the cortex formed on the gel surface as ...
Embodiment 2
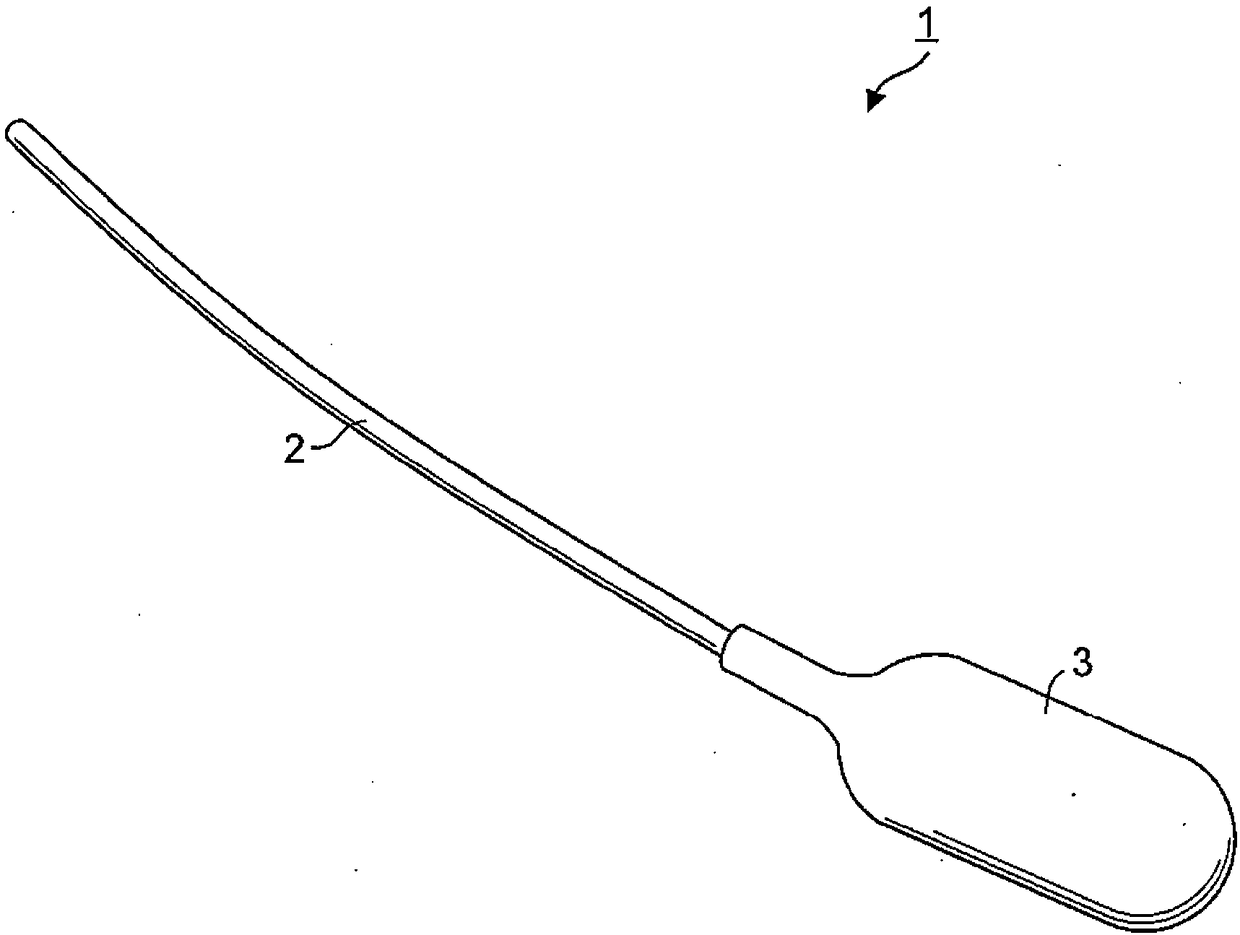
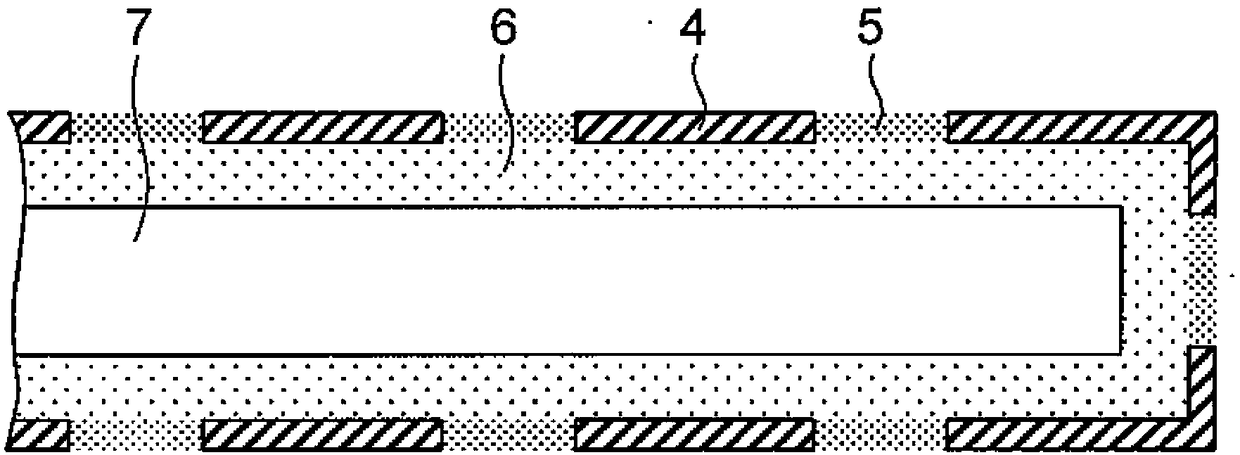
[0112] One hollow fiber used in a polysulfone membrane dialyzer (APS-15SA4537693003682) manufactured by Asahi Kasei Medical Co., Ltd. was used as the device (inner diameter: 185 μm, membrane thickness: 45 μm). In this example, the device was connected to a commercially available human silicone catheter (4Fr: about 600 μm inner diameter, Primetech Co., Ltd.), which served as a reservoir for supplying insulin.
[0113] Insulin release experiments were performed using a high performance liquid chromatography (HPLC) system (JASCO, Japan) with 2 pumps and internal detectors for detection of refractive index (RI), UV, fluorescence intensity.
[0114] The gel prepared in the same manner as in Example 1 was soaked in PBS containing 130 mg / L FITC-labeled bovine insulin (WAKO, Japan) at 4° C. for 24 hours to encapsulate FITC-labeled insulin in the gel. Next, the gel was filled into the device of the present invention, and the device was quickly placed in a 0.01M hydrochloric acid soluti...
Embodiment 3
[0119] Using the same system as in Example 2, the device used is made by sealing the two ends of 10 hollow fibers. Used. In this example, no reservoir is used.
[0120] The result is as Figure 5 As shown, it was confirmed that as the concentration of glucose in the outer fluid of the hollow fiber increased, the fluorescence intensity increased, and insulin was released from the device. When the glucose concentration decreases, the release of insulin is inhibited.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com