A kind of silver vanadate nano belt and preparation method thereof
A technology of nanobelt and silver vanadate, which is applied in the direction of nanotechnology, chemical instruments and methods, vanadium compounds, etc., can solve the problems of restrictive conditions, many steps, and high cost, and achieve simple operation, low cost, and mild and reliable hydrothermal conditions. control effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0047] This embodiment proposes a method for preparing silver vanadate nanobelts, comprising the following steps:
[0048] 1. Add the vanadium-containing compound into the dispersant, and fully disperse the vanadium-containing compound by stirring or vibrating to form a suspension A.
[0049] In this embodiment, vanadium pentoxide is selected as the vanadium-containing compound, ultrapure water is selected as the dispersant, and silver nitrate is selected as the silver-containing compound. The ratio of vanadium pentoxide to ultrapure water is 0.3-3mmol:15ml.
[0050] 2. According to the molar ratio of silver in the silver-containing compound to vanadium in the vanadium-containing compound is 0.5-1:1, add a certain amount of silver-containing compound to the suspension A, transfer it to the dark box, and use it under vigorous stirring. The silver-containing compound is fully dissolved to form a suspension B, which is stored in a dark place.
[0051] 3. Put the suspension B at...
Embodiment 1
[0067] Such as figure 1 Shown, embodiment 1 proposes a kind of preparation method of silver vanadate nanobelt, comprises the following steps:
[0068] 1. Add 0.3mmol of vanadium pentoxide to 15ml of ultrapure water, and use electromagnetic stirring to fully disperse the vanadium pentoxide to form a suspension A.
[0069] 2. According to the molar ratio of silver in silver nitrate and vanadium of vanadium pentoxide is 1:1, add a certain amount of silver nitrate into the suspension A, transfer it to the dark box, and make the silver nitrate fully Dissolve to form Suspension B, and store in shading.
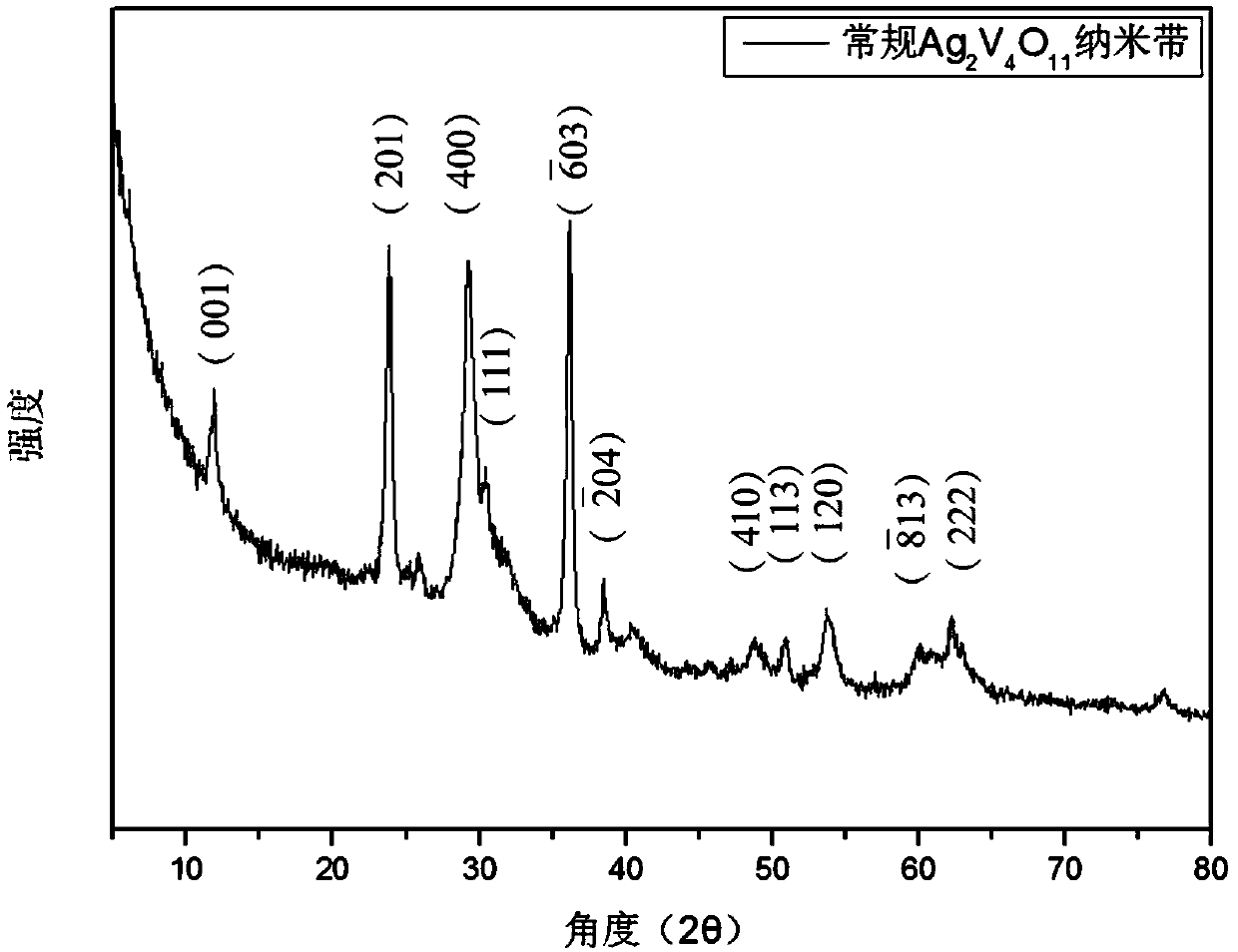
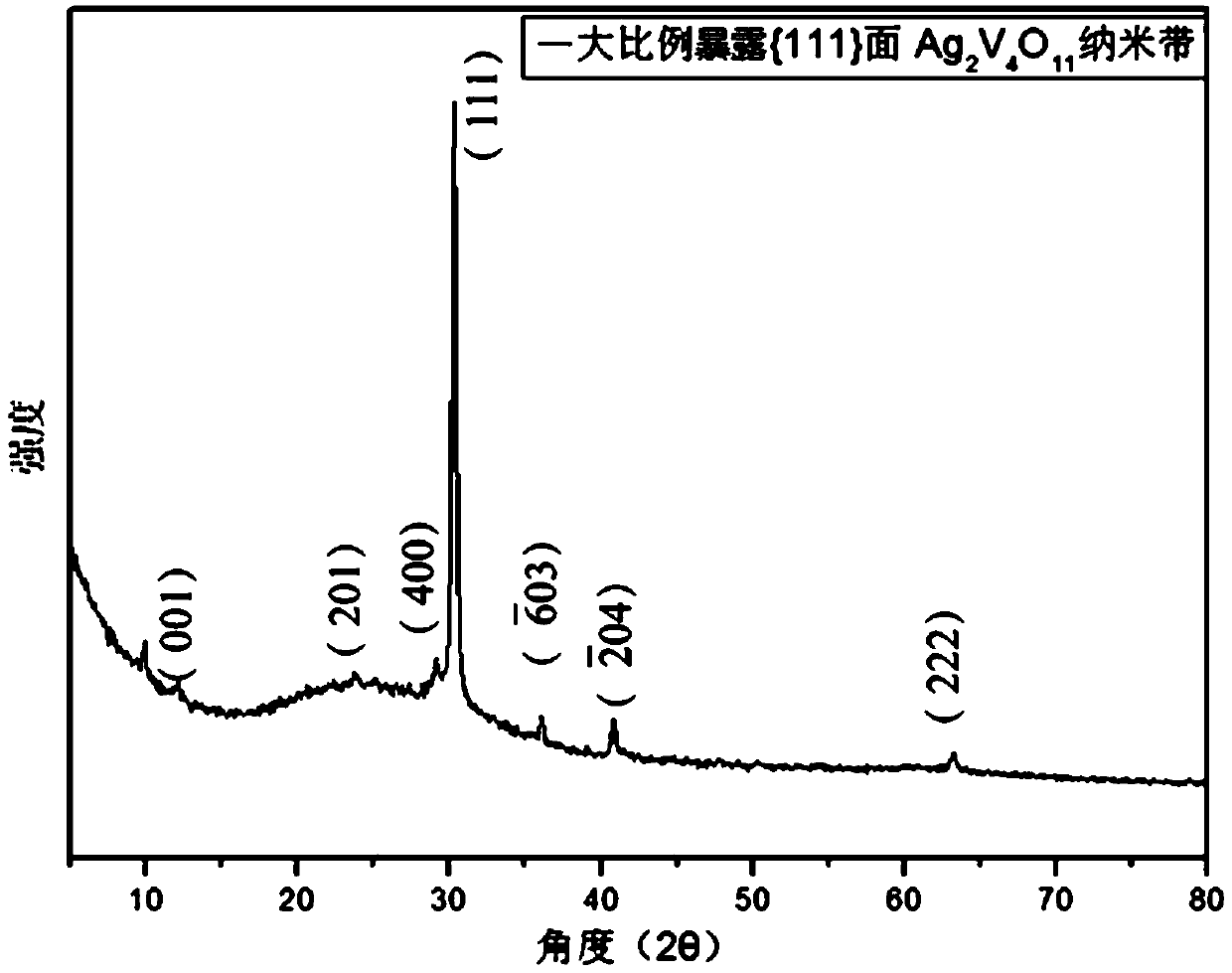
[0070] 3. Move the suspension B to a high-pressure reactor, seal it, and heat it at 120°C for 48 hours. After high-temperature hydrothermal treatment, a dark green Ag 2 V 4 o 11 Precipitated product mixture.
[0071] 4. Centrifuge the product mixture in step 3 for 10 minutes at a speed of 3800 rpm in a centrifuge, remove the supernatant, collect the precipitate, and then repeat...
Embodiment 2
[0074] Embodiment 2 proposes a kind of preparation method of silver vanadate nanobelt, comprises the following steps:
[0075] 1. Add 1mmol of vanadium pentoxide to 15ml of ultrapure water, and use a vibrating sieve to fully disperse the vanadium pentoxide to form a suspension A.
[0076]2. According to the molar ratio of silver in silver nitrate and vanadium pentoxide of 0.8:1, add a certain amount of silver nitrate into the suspension A, transfer it to the dark box, and make the silver nitrate fully Dissolve to form Suspension B, and store in shading.
[0077] 3. Move the suspension B to a high-pressure reactor, seal it, and heat it at 150°C for 48 hours. After high-temperature hydrothermal treatment, a dark green Ag 2 V 4 o 11 Precipitated product mixture.
[0078] 4. Centrifuge the product mixture in step 3 for 10 minutes at a speed of 3800 rpm in a centrifuge, remove the supernatant, collect the precipitate, and then repeatedly wash the precipitate with deionized wate...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| width | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com