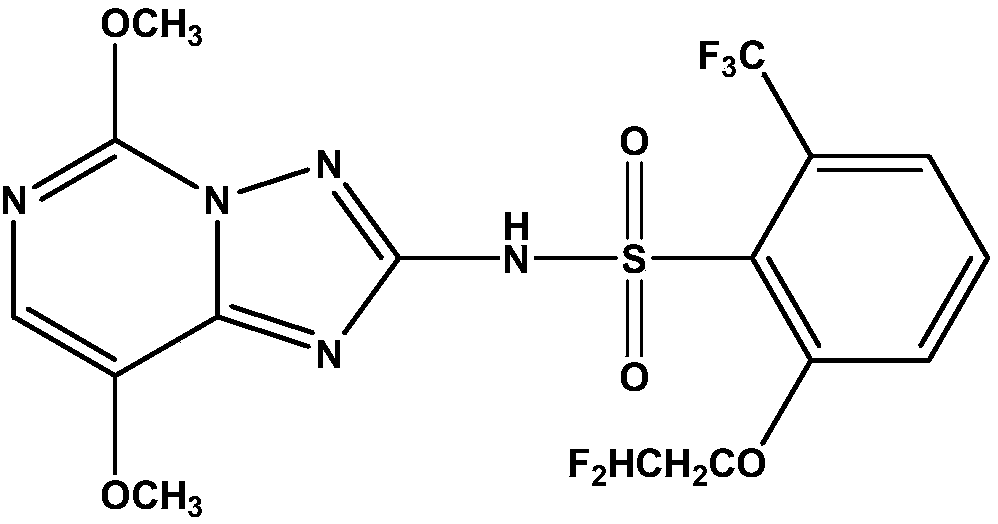
Triazolopyrimidine sulfonamide compound as well as preparation method and application thereof
A technology for azolopyrimidine sulfonamides and compounds, which is applied in the field of triazolopyrimidine sulfonamide compounds and their preparation, can solve the problems of penoxsulam complex structure, difficult synthesis, and high cost, and achieve simple and easy preparation methods Operation, strong selectivity, low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0034] One embodiment of the present invention provides a method for preparing triazolopyrimidine sulfonamide compounds, comprising the following steps:
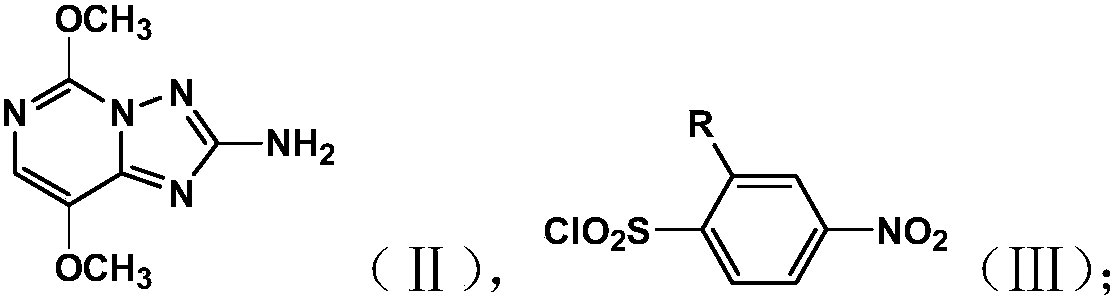
[0035] Take the compound A whose structural formula is shown in formula (II) and the compound B whose structural formula is shown in formula (III);
[0036]
[0037] Wherein, R is a chlorine atom or a methoxycarbonyl group;
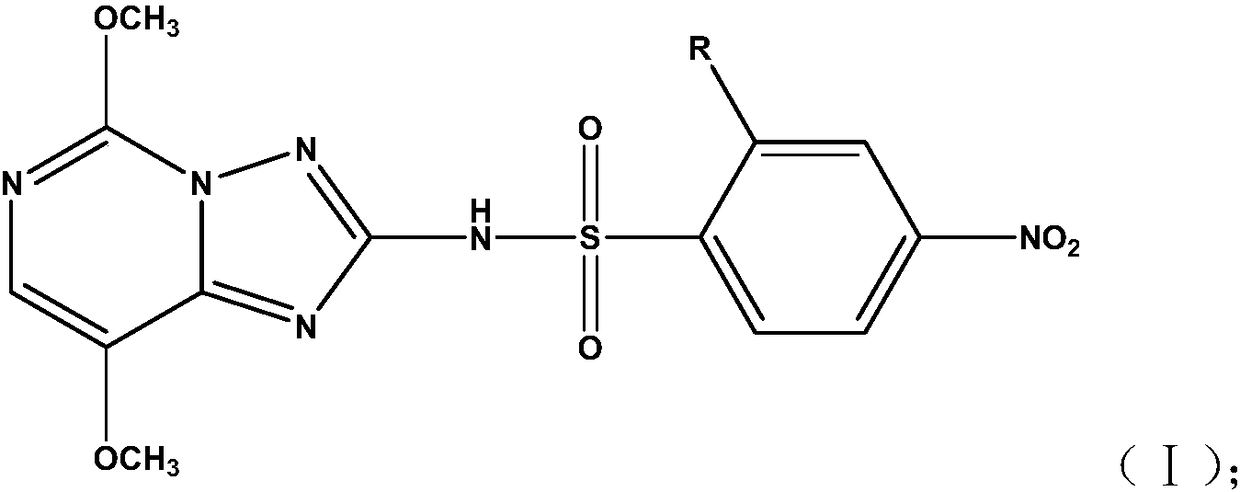
[0038] The compound A and the compound B are added into an organic solvent according to a molar ratio of 1:(1.1-1.4), and reacted at room temperature for 18-24h in the presence of a catalyst to obtain a structural formula as shown in formula (I) Triazolopyrimidine sulfonamide compounds,
[0039]
[0040] Wherein, R is a chlorine atom or a methoxycarbonyl group.
[0041] The preparation method chemical equation of compound I involved in the present invention is as follows:
[0042]
[0043] Optionally, the molar ratio of the compound A to the compound B is 1:1.2.
[0044] Optionally, the cat...
Embodiment 1
[0061] The synthetic process of N-(4,7-dimethoxy[1,2,4]triazolo[1,5-c]pyrimidine)-2-chloro-4-nitrobenzenesulfonamide (I-a) is as follows:
[0062] Add 3.0g 4,7-dimethoxy[1,2,4]triazolo[1,5-c]pyrimidineamino, 4.8g 2-chloro-4-nitrobenzenesulfonyl chloride, 30mL in a 100mL flask 3,5-lutidine, stirred slowly mechanically, then added 2 drops of DMSO, and reacted at room temperature for 24h.
[0063] After the reaction was completed, the above-mentioned resulting product was filtered with filter paper, rinsed with a small amount of deionized water, then the filter residue was scraped into another 100mL round-bottomed flask, and 30mL of 15% sulfuric acid was added to the round-bottomed flask (3.6g was added in 36.4mL of water) 15% sulfuric acid) and 10 mL of acetonitrile, and continued mechanical stirring for 30 min. At this time, the color changed from dark orange to white, and the reaction process was monitored by TLC. The volume ratio of the developer is: ethyl acetate:methanol=1...
Embodiment 2
[0067] N-(4,7-dimethoxy[1,2,4]triazolo[1,5-c]pyrimidine)-2-methoxycarbonyl-4-nitrobenzenesulfonamide (I-b) synthesis process is as follows :
[0068] Add 2g 4,7-dimethoxy[1,2,4]triazolo[1,5-c]pyrimidineamino, 3.35g 2-methoxycarbonyl-4-nitrobenzene in a 100ml single-necked round bottom flask Sulfonyl chloride, 20ml 3,5-lutidine, add 2 drops of DMSO, react at room temperature for 18h.
[0069] After the reaction is complete, filter the above-mentioned product with filter paper, rinse with a small amount of deionized water, then add the filter residue to another 100mL round-bottom flask, add 30mL of 15% sulfuric acid and 10mL of acetonitrile to the round-bottom flask, and continue stirring for 30min , the color changed from dark orange to white at this time, and the reaction process was monitored by TLC. The developer ratio is: ethyl acetate: methanol = 10:1. After the reaction was completed, it was filtered with suction to obtain a white solid, which was separated by silica g...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com