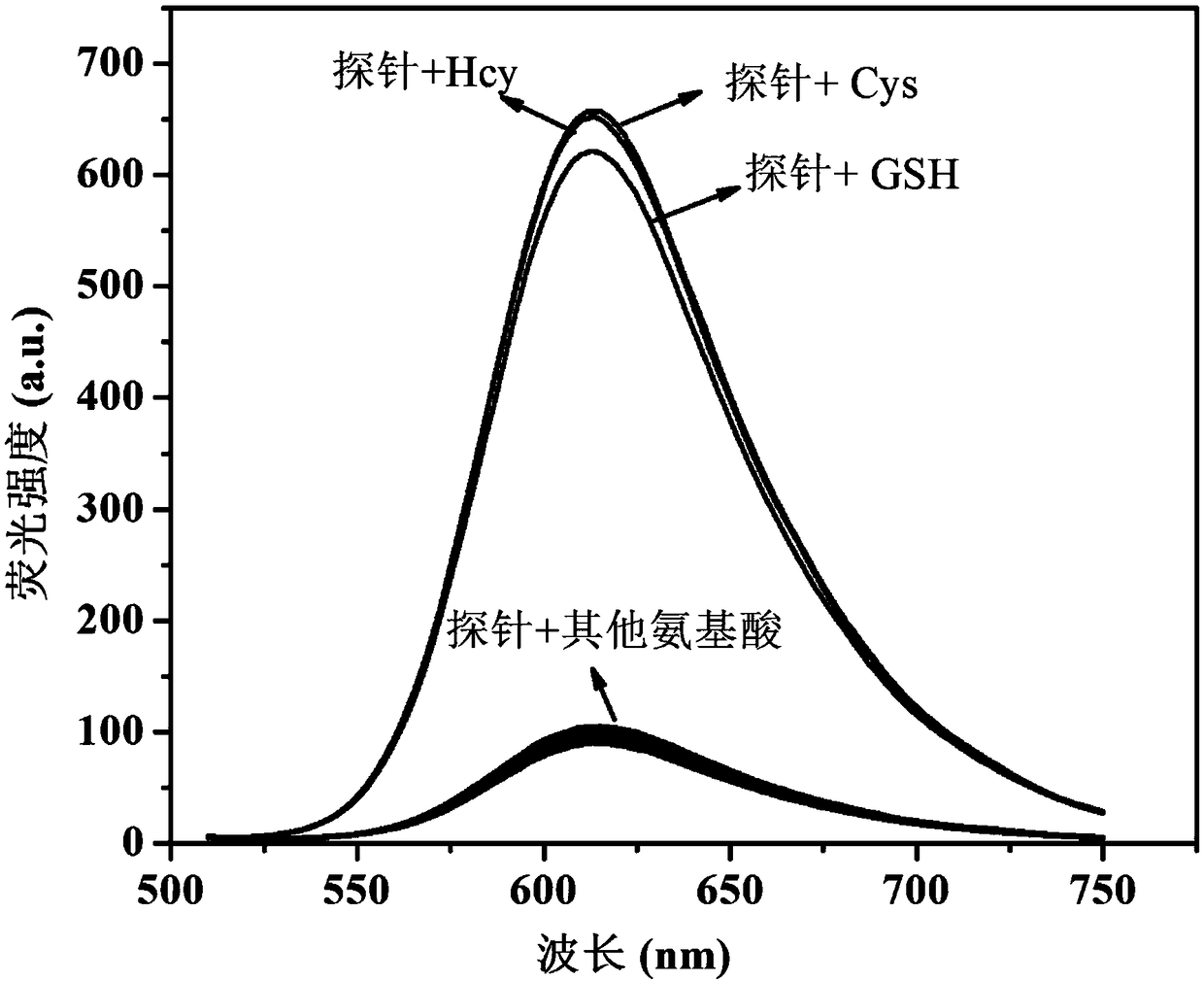
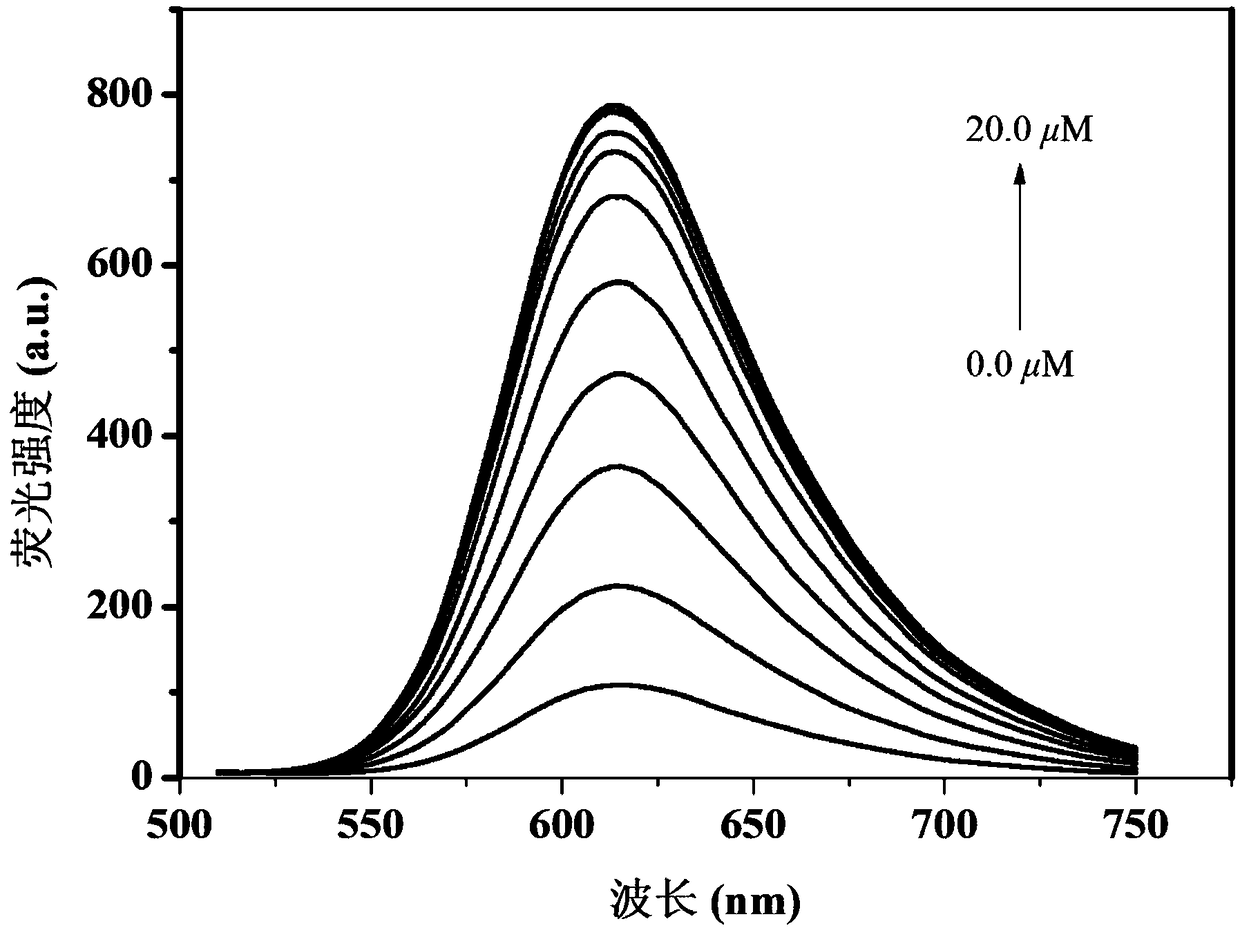
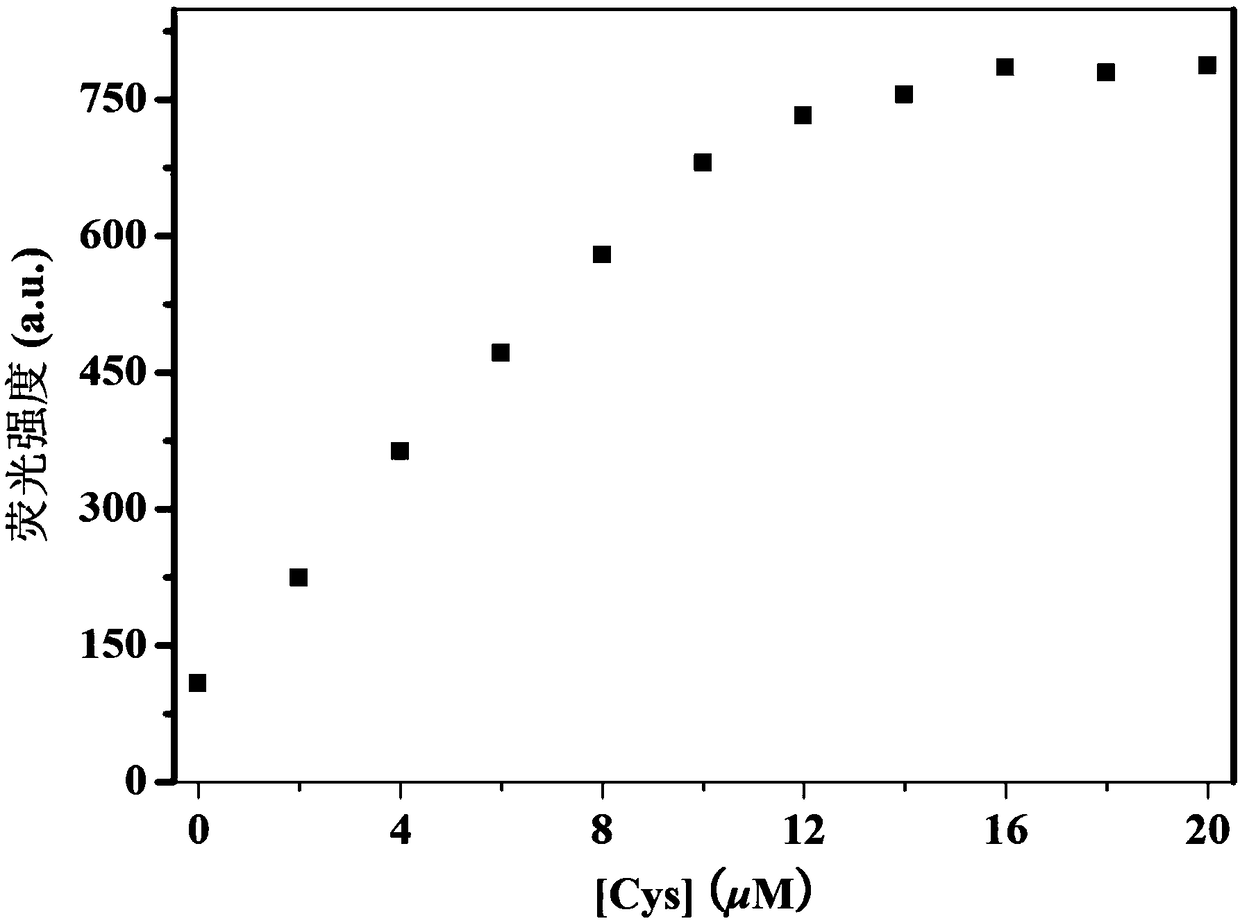
Fluorescent probe for detecting biological thiols
A biological thiol and fluorescent probe technology, applied in the field of fluorescent probes, can solve the problems of expensive instruments and equipment, cumbersome processing, complicated operation process, etc., and achieve the effects of low cost and simple synthesis route.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Embodiment 1: the synthesis of intermediate product 2
[0026] Add 10.0mL acetone to a 50mL single-necked round bottom flask, then dissolve compound 1 (0.2173g, 1.0mmol) and 3-bromopropyne (0.2380g, 2.0mmol) in acetone, then add anhydrous potassium carbonate (0.2764g , 2.0mmol), then heated to reflux for 12 hours, stopped the reaction, filtered the reaction solution to remove the filter residue, spin-dried to obtain a solid crude product, and finally column chromatography (silica gel 200-300 mesh, eluent: V 乙酸乙酯 / V 石油醚 =1 / 3) 0.2002 g of yellow solid was isolated with a yield of 78.4%. 1 H NMR (500MHz, CDCl 3 )δ H :10.03(s,1H),7.34(s,1H),4.63(d,J=2.4Hz,2H),3.52-3.05(m,4H),2.81(t,2H),2.73(t,2H), 2.55(t,1H),2.01-1.82(m,4H). 13 C NMR (125MHz, CDCl 3 )δ C :187.8,157.8,148.8,127.5,117.5,117.0,112.6,78.7,76.1,62.1,50.0,49.7,27.3,21.4,21.3,20.7.
Embodiment 2
[0027] Embodiment 2: the synthesis of intermediate product 3
[0028] Compound 2 (0.2552g, 1.0mmol) and 4-aminophenol (0.1308g, 1.2mmol) were added to a 25mL single-necked round bottom flask containing 5.0mL of anhydrous DMF, reacted at 110°C for 4h, stopped the reaction, and cooled to room temperature. The reaction solution was poured into 50 mL saturated brine, extracted with dichloromethane (25.0 mL×4), washed with saturated brine, dried over anhydrous sodium sulfate, and spin-dried under reduced pressure to obtain a crude product, which was then subjected to column chromatography (eluent: V 石油醚 / V 乙酸乙酯 =6 / 1 to 3 / 1) isolated the product 0.0689 g; yield 20%. 1 HNMR (400MHz, DMSO-d 6 )δ H9.85(s,1H),7.78(d,J=9.2Hz,2H),7.65(s,1H),7.22(dd,J=9.0,2.6Hz,1H),7.06(d,J=2.6Hz,1H ),5.19(s,2H),3.19–3.06(m,4H),2.72(t,J=6.2Hz,2H),2.60(t,J=6.4Hz,2H),2.52-2.47(m,1H) ,1.86(m,4H). 13 C NMR (100MHz, DMSO-d 6 )δ C 155.0, 153.7, 147.2, 145.6, 143.3, 130.0, 129.2, 128.2, 125.2, 122.4, 121...
Embodiment 3
[0029] Embodiment 3: the synthesis of intermediate product 4
[0030] Add compound 3 (0.1801g, 0.52mmol) and 5mL of anhydrous dichloromethane into a 20mL thick-walled pressure bottle, then add iodomethane (0.0863g, 0.6mmol), react at 90°C for 12h in the dark, and stop the reaction. Cool to room temperature, remove solvent under reduced pressure to obtain crude product, finally through column chromatography (eluent: V 二氯甲烷 / V 甲醇 =50 / 1 to 33 / 1) isolated the product 0.0932 g, yield 87%. 1 H NMR (500MHz, DMSO-d 6 )δ H 10.64(s,1H),8.45(s,1H),8.12(d,J=9.3Hz,1H),7.61-7.42(m,2H),7.36(s,1H),5.19(s,2H),4.35 (s,3H),2.72(d,J=49.8Hz,4H),1.92-1.89(m,4H),1.40-1.12(m,4H). 13 C NMR (400MHz, DMSO-d 6 )δ C 157.5,157.0,149.2 147.7,135.3135.0,130.5,128.7,127.8,124.5,121.2,117.2,111.3,107.5,103.3,67.9,50.1,49.5,44.5,27.3,21.3,203.6,2
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com