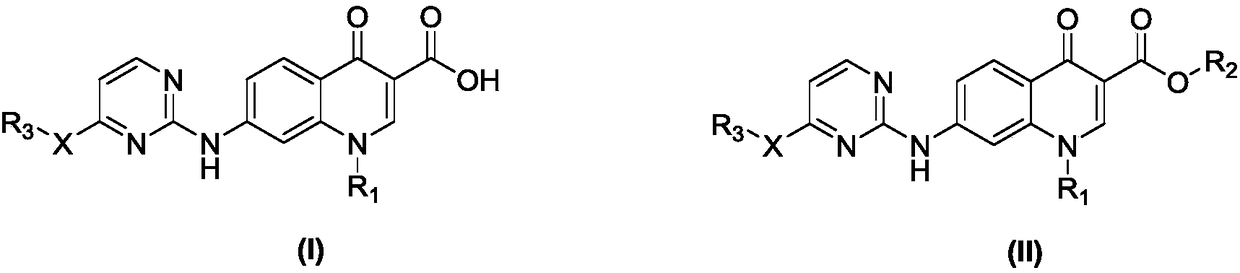
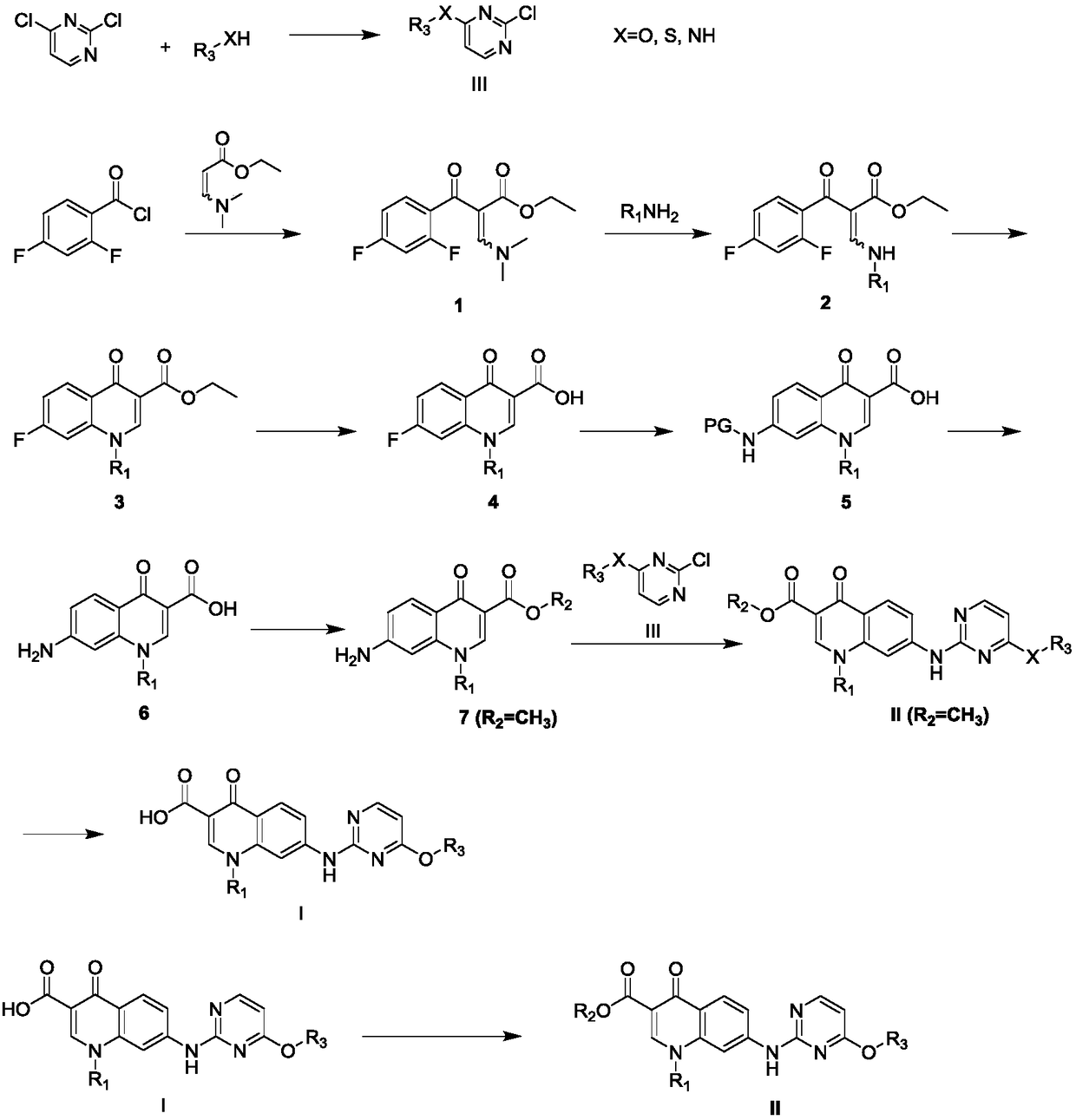
Pyrimidine-quinolone hybrids, and preparation method and application thereof
A technology of quinolones and hybrids, which is applied in the field of pyrimidine-quinolones hybrids and their preparation, can solve the problems of drug toxicity and side effects limiting clinical application, and achieve good broad-spectrum anti-tumor activity, novel structure, and good antibacterial activity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
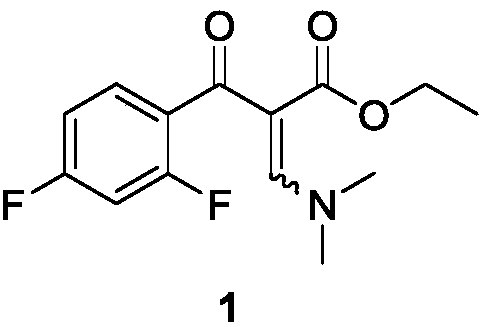
[0044] Embodiment 1: the preparation of intermediate 1
[0045]
[0046]Dissolve 2,4-difluorobenzoyl chloride (18.1mmol), ethyl 3-(N,N-dimethylamino)acrylate (18.1mmol), and triethylamine (27.1mmol) in 30mL of toluene, and stir at 90°C for 4h , concentration, and column chromatography to obtain intermediate 1. Yield 65%, 1 H NMR (400MHz, CDCl 3 )δ0.95-0.99(t, J=7.2Hz, 3H), 2.89(s, 3H), 3.31(s, 3H), 3.98-4.03(q, J=7.2Hz, 2H), 6.74-6.80(m ,1H), 6.89-6.93(m,1H), 7.62-7.68(m,1H), 7.79(s,1H).
Embodiment 2
[0047] Embodiment 2: the synthesis of intermediate 4
[0048]
[0049] Intermediate 1 (3mmol) and various substituted amines (3.6mmol) were stirred in THF (5mL) at 50°C for 3h and concentrated to obtain Intermediate 2, which was directly used in the next reaction without separation and purification.
[0050] Intermediate 2 (1.5mmol), K 2 CO 3 (2.3mmol) was added to DMF (10mL), stirred overnight at 60-90°C, cooled, poured into ice water, filtered, washed with water to obtain intermediate 3, which was directly used in the next reaction without separation and purification.
[0051] Dissolve intermediate 3 (1 mmol) in THF (3 mL), add 10% NaOH aqueous solution (3 mL), stir at 50 °C for 0.5-5 h, cool, concentrate, adjust pH to 2 with 5% hydrochloric acid, filter, wash with water, Intermediate 4 was obtained by drying.
[0052] 4a(R 1 =t-Butyl): yield 70%; 1 H NMR (400MHz, DMSO-d 6 )δ14.81(s,1H),9.13(s,1H),8.66(dd,J=9.0,6.8Hz,1H),7.74(dd,J=11.8,2.2Hz,1H),7.32(td,J =7.3,2.2H...
Embodiment 3
[0056] Embodiment 3: the synthesis of intermediate 6
[0057]
[0058] Intermediate 4 (1.0 mmol) was dissolved in DMSO (2 mL), 2,4-dimethoxybenzylamine (3.0 mmol) was added, N 2 React at 85°C for 6 h under protection, pour into ice water after cooling, and adjust the pH to about 5 with 6N HCl, filter, wash with ethanol, and dry to obtain intermediate 5, which is directly used in the next step without further purification.
[0059] Intermediate 5 (5.0 mmol) was dissolved in dichloromethane (30 mL), trifluoroacetic acid (15.0 mmol) was added, and stirred at room temperature for 5 h. After the reaction was completed, poured into ice water (250 mL) and stirred vigorously for 20 min, and filtered. After the filter cake was dried, it was poured into dichloromethane (500 mL) and sonicated for 10 min, and the insoluble matter was removed by filtration, and the dichloromethane was removed from the filtrate under reduced pressure to obtain intermediate 6.
[0060] Intermediate 6 (1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com