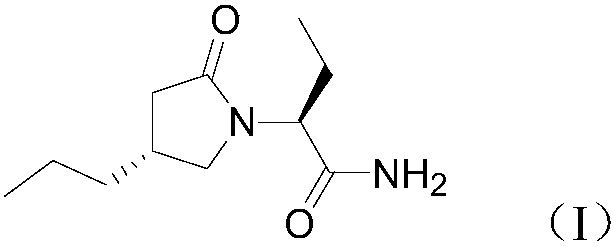
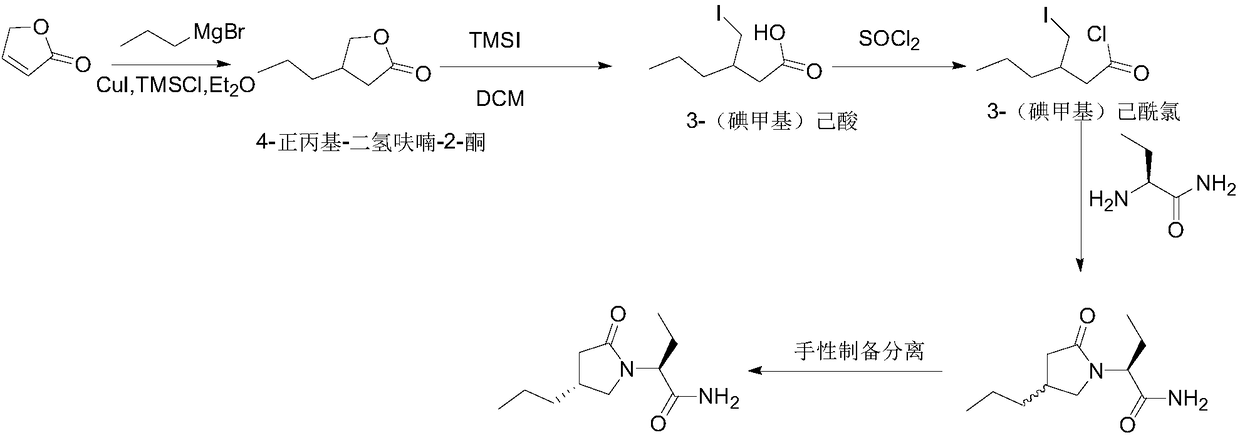
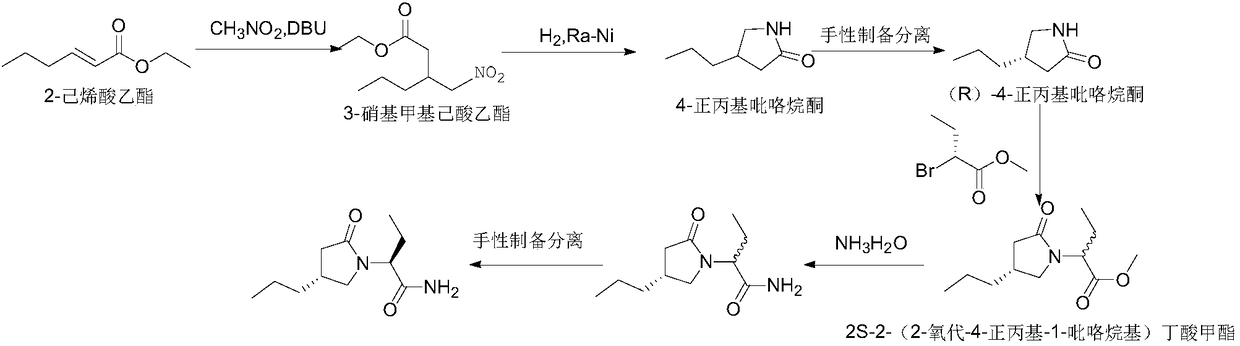
New preparation method of brivaracetam
A compound and molar ratio technology, applied in a new field of preparation, can solve problems such as high cost and unsuitable for large-scale production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0089] Embodiment 1: the synthesis (toluene as solvent) of (R)-3-bromomethylhexanoic acid
[0090]
[0091] operate:
[0092] At room temperature, dry toluene (60mL) was added to a 250mL three-necked flask, and (R)-4-n-propyl-dihydrofuran-2(3H)-one (12.8g, 0.1mol, 1eq) and no Zinc chloride hydrate (6.8g, 0.05mol, 0.5eq) was added dropwise with trimethylbromosilane (61.2g, 0.4mol, 4eq) under stirring. After the addition was completed, the temperature was raised to 70-80°C for 1 hour. As detected by TLC, (R)-4-n-propyl-dihydrofuran-2(3H)-one disappeared, the heating was stopped and the temperature was lowered. Water (100 mL) was added dropwise at 0-10°C to quench the reaction. Separate the layers, wash the organic phase with water (100 mL×2), and wash once with saturated sodium chloride (100 mL) solution, collect the organic phase, and dry over anhydrous sodium sulfate (10 g) for 2 hours. Filter and concentrate the organic phase to dryness. 18.1 g of the target compound w...
Embodiment 2
[0094] Example 2: Synthesis of (R)-3-bromomethylhexanoic acid (n-heptane as solvent)
[0095] operate:
[0096]At room temperature, dry n-heptane (60mL) was added to a 250mL three-necked flask, followed by (R)-4-n-propyl-dihydrofuran-2(3H)-one (12.8g, 0.1mol, 1eq) and anhydrous zinc chloride (6.8g, 0.05mol, 0.5eq), under stirring, trimethylbromosilane (61.2g, 0.4mol, 4eq) was added dropwise, after the addition was complete, the temperature was raised to 70-80°C for 1 hour. As detected by TLC, (R)-4-n-propyl-dihydrofuran-2(3H)-one disappeared, the heating was stopped and the temperature was lowered. Water (100 mL) was added dropwise at 0-10°C to quench the reaction. Separate the layers, wash the organic phase with water (100 mL×2), and wash once with saturated sodium chloride (100 mL) solution, collect the organic phase, and dry over anhydrous sodium sulfate (10 g) for 2 hours. Filter and concentrate the organic phase to dryness. 19.4 g of the target compound was obtained a...
Embodiment 3
[0097] Embodiment 3: the synthesis of (R)-3-bromomethylhexanoyl chloride
[0098]
[0099] operate:
[0100] At room temperature, dichloromethane (100mL) was added to a 250mL three-necked flask, (R)-3-bromomethylhexanoic acid (19.0g, 0.09mol, 1eq) was added, and under stirring, thionyl chloride ( 32.1 g, 0.27 mol, 3 eq). After addition, the reaction was stirred at room temperature. TLC detects that the starting material disappears, and the reaction is stopped. Concentrate to dryness under reduced pressure to obtain 21.4 g of the target compound as a yellow oil with a yield of 104.5%. used directly in the next step.
[0101] 1 H NMR (400MHz, Chloroform-d) δ3.58 (ddd, J = 18.6, 10.5, 3.9Hz, 1H), 3.52–3.42(m, 1H), 3.20–2.87(m, 1H), 2.73–2.36(m ,1H),2.33–2.14(m,1H),1.53–1.26(m,4H),0.98–0.89(m,3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com