Preparation method of azoxystrobin intermediate
An intermediate, azoxystrobin technology, applied in the field of preparation of important intermediates, can solve the problems of incomplete conversion of raw materials, high cost of raw materials, low total yield, etc., to improve the total yield of the reaction, simplify the production process, raw materials low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
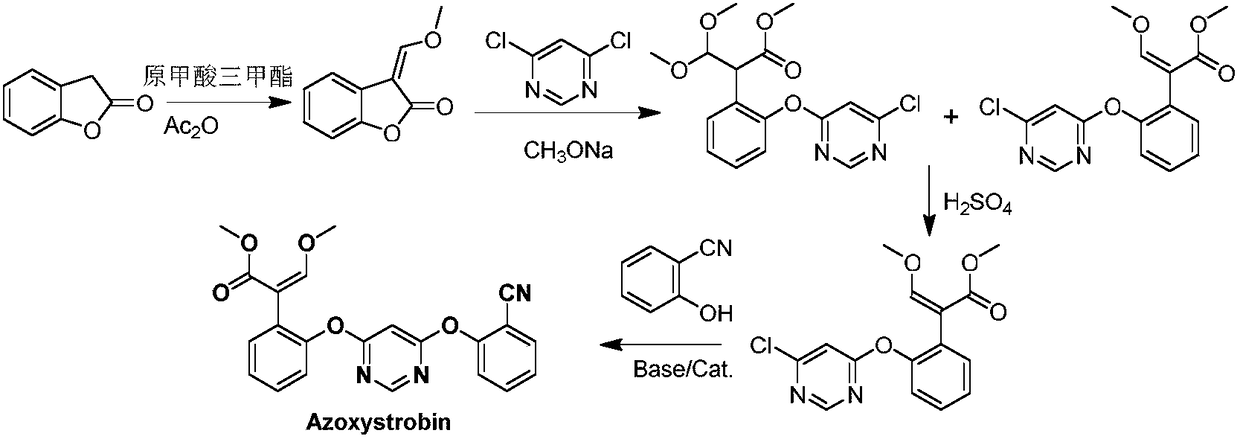
[0036] Embodiment 1: the preparation of N,N-dimethylformamide dimethyl acetal
[0037]
[0038] The reaction is as above, add 289g (3.96mol) of DMF into the reaction flask, heat and stir, add 450g (3.57mol) of dimethyl sulfate dropwise at a controlled temperature of 65°C, add it for about 1 hour, stir, and react at a controlled temperature of 75°C for 3h to obtain The imine complex was cooled in an ice bath to below 0°C for use. Add 771g (4.28mol) of liquid sodium methoxide into the reaction flask, distill off the methanol, add 1400mL of petroleum ether (45°C), stir vigorously to disperse the sodium methoxide, control the temperature at 0°C, and add the above imine complex dropwise. After about 2 hours of addition, the temperature was controlled at 0°C and the reaction was stirred for 2 hours, and then filtered. The filtrate is fractionated with a fractionating column (L=30cm, ф=2cm, metal ring packing) under normal pressure, and 344g of fractions at 105-106°C are collecte...
Embodiment 2
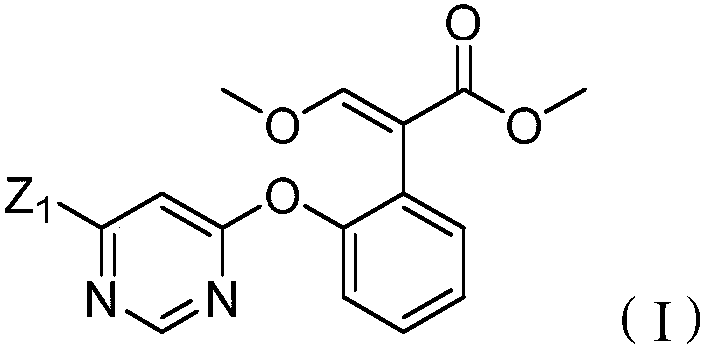
[0039] Example 2 Preparation of methyl 2-[2-(6-chloropyrimidine-4-oxyl)phenyl]-3-dimethylaminoacrylate (i.e. in formula (Ⅲ), Z 1 is chlorine, R 1 , R 2 both methyl)
[0040] In a four-neck flask equipped with a mechanical stirrer, a thermometer, and a condenser tube, add 29.5g (0.1mol, 95%) 2-(2-(6-chloropyrimidine-4-oxyl)phenyl)methyl acetate, 17.8 g The N,N-dimethylformamide dimethyl acetal (0.15mol) prepared in Example 1 and 150mL of toluene were stirred and heated to 85°C, the low boiling point compound methanol was distilled off, and kept for 10h; after the reaction, the temperature was lowered Wash and separate layers, and concentrate the organic phase to obtain a solid that is methyl 2-(2-(6-chloropyrimidine-4-oxyl)phenyl)-3-dimethylaminoacrylate, which is directly carried out to the next reaction without separation. The yield of methyl 2-(2-(6-chloropyrimidine-4-oxyl)phenyl)-3-dimethylaminoacrylate was measured to be 98%.
Embodiment 3
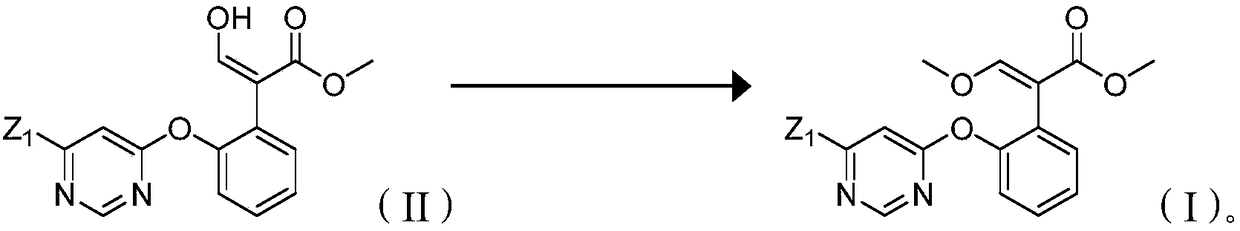
[0041]Example 3: Preparation of 2-[2-(6-chloropyrimidin-4-yloxy)phenyl]-3-methoxymethyl acrylate (i.e. in formula (II), Z 1 is chlorine, the structure is shown below)
[0042]
[0043] 34.0 g (content 98%) of 2-(2-(6-chloropyrimidine-4-oxyl)phenyl)-3-dimethylaminoacrylate methyl ester provided in Example 2 was dropped into methanol and dropped at 25° C. Add 40.5 g (0.2 mol) of 18% hydrochloric acid aqueous solution, stir at room temperature for 3 h, then distill off methanol under reduced pressure, add dichloromethane for extraction, leave to separate layers, separate the water layer, add 40 g of 20% sodium hydroxide (0.2 mol) to the organic layer ) aqueous solution, 15.2g dimethyl sulfate (0.12mol) was added dropwise, stirred at room temperature for 3h, the reaction was completed, the water layer was separated after standing, and the organic phase was concentrated to obtain the target product 2-(2-(6-chloropyrimidine-4-oxyl ) phenyl)-3-methoxymethyl acrylate (ie azoxystro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com