Preparation method of methyl hydroquinone/2-methylhydroquinone as novel polymerization inhibitor
A technology of methyl hydroquinone and methyl hydroquinone, applied in the field of preparation of methyl hydroquinone, can solve problems such as unfavorable industrial production, low reaction yield, harsh reaction conditions and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
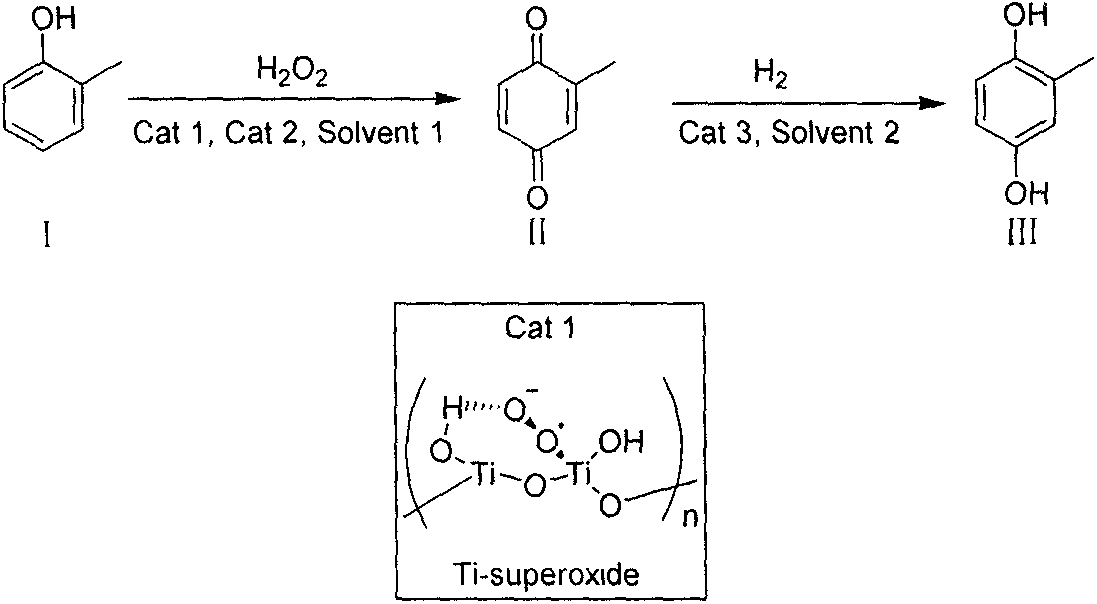
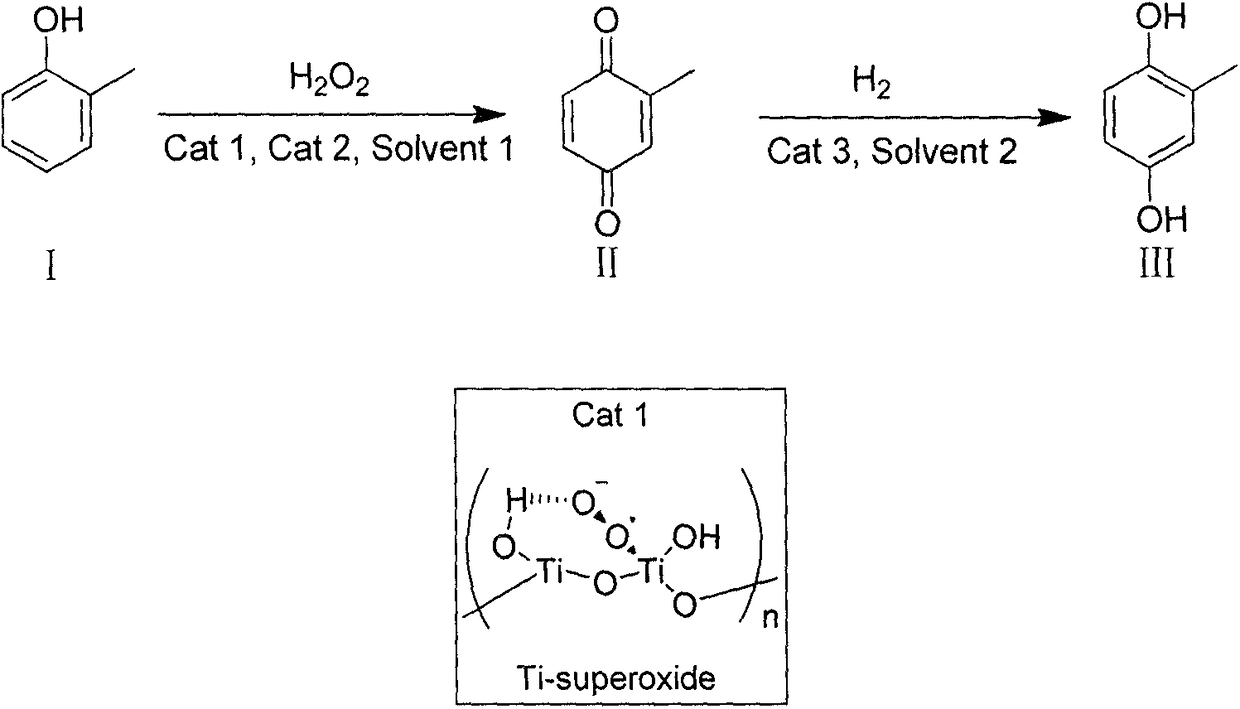
[0034]Add 40.0g o-cresol, 20% (w / w) catalyst Ti-Superoxide 8.0g and 1% (w / w) benzyltriethylammonium chloride in a 500mL eggplant-shaped flask, and heat under strong mechanical stirring 320 mL of 30% hydrogen peroxide (99% conversion, 91% selectivity) was slowly added dropwise under the condition of maintaining the reaction system at 50-60°C. After reacting for 1 hour, cool to room temperature, filter and wash the catalyst three times with 300mL toluene and extract the reaction solution, recover the catalyst and hydrogen peroxide for recycling, combine the organic phases and directly put them into a closed container, add 4.5g Raney nickel, at 0.6MPa, 100 °C under hydrogen pressure for more than 10 hours. After the reaction was completed, the catalyst was removed, heated to reflux and allowed to stand for 5 minutes, the upper layer of toluene solvent was poured out and cooled to crystallize to obtain an off-white solid, which was then recrystallized from toluene to obtain 23.0 g...
Embodiment 2
[0036] Add 40.0g o-cresol, 20% (w / w) catalyst Ti-Superoxide and 1% (w / w) tetrabutylammonium bromide into a 500mL eggplant-shaped flask, and heat to 50-60°C under strong mechanical stirring. ℃, and slowly drop 320 mL of 30% hydrogen peroxide (99% conversion, 91% selectivity) under the condition of maintaining the reaction system at 50-60 ℃. After reacting for 1 hour, cool to room temperature, filter and wash the catalyst with 300mL toluene and extract the reaction solution, recover the catalyst and hydrogen peroxide for recycling, combine the organic phase and put it directly into a closed container, add 4.5g of Raney nickel at 0.6MPa, hydrogen at 100°C Reaction under pressure for more than 10 hours. After the reaction was completed, the catalyst was removed, heated to reflux and then allowed to stand for 5 minutes. The upper layer of toluene solvent was poured out while it was hot and cooled to crystallize to obtain an off-white solid, which was then recrystallized with toluen...
Embodiment 3
[0038] In a 500mL eggplant-shaped flask, add 40.0g o-cresol, 20% (w / w) catalyst Ti-Superoxide 8.0g and 1% (w / w) benzyltriethylammonium chloride 0.4g and 200mL benzene, in strong Heated to 50-60° C. under mechanical stirring, and slowly added dropwise 320 mL of 30% hydrogen peroxide (99% conversion, 91% selectivity) under the condition of maintaining the reaction system at 50-60° C. After reacting for 1 hour, cool to room temperature, filter and wash the catalyst with 100mL benzene and extract the reaction liquid, recover the catalyst and hydrogen peroxide for recycling, combine the organic phase and put it directly into a closed container, add 4.5g of Raney nickel at 0.6MPa, hydrogen at 100°C Reaction under pressure for more than 10 hours. After the reaction was completed, the catalyst was removed, heated to reflux and then allowed to stand for 5 minutes. The upper layer of benzene solvent was poured out while it was hot and cooled to crystallize to obtain an off-white solid, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com