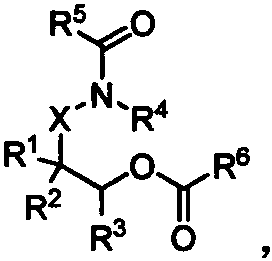
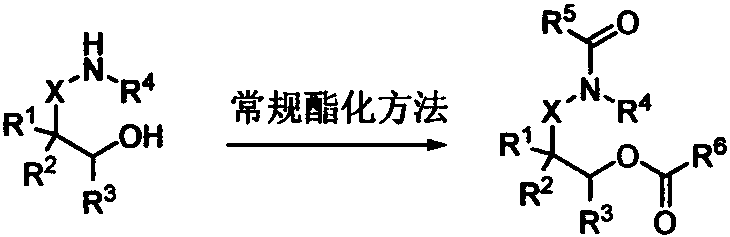
Method using amide ester to prepare diamine derivative
A technology of diamine derivatives and amide esters, which is applied in the field of preparing diamine derivatives from amide esters, can solve complex problems, achieve the effects of low dosage, simple reaction conditions, and high stereoselectivity of products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-7
[0036] A method for preparing diamine derivatives by amide ester, the specific steps are:
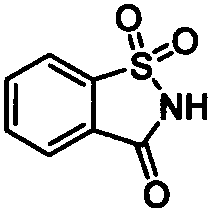
[0037] Add 145mg 2-acetamidoethanol acetate 1a, 91.5mg saccharin 2 successively in 5mL sample bottle (the raw material feeding amount is fixed as 1a: 2=2: 1), different trifluoromethanesulfonate catalysts in Table 1, and chlorobenzene (1.0M) solvent. Seal and heat to 120°C, and under the condition of constant temperature of 120°C, stir for substitution reaction for 12 hours to prepare ortho diamine derivative 3a, one of the diamine groups of the diamine derivative is retained from amide Carboxylic acid amides of ester raw materials. 1 H NMR (500MHz, CDCl 3 )δ=8.08(d, J=7.4Hz, 1H), 7.98-7.84(m, 3H), 6.17(s, 1H), 3.96-3.91(m, 2H), 3.65(dd, J=10.9, 5.7Hz , 2H), 1.98(s, 3H). 13 C NMR (126MHz, CDCl 3 )δ=170.7, 159.3, 137.4, 135.1, 134.6, 127.1, 125.3, 121.1, 39.5, 38.1, 23.2. HR-MS (ESI-TOF) calcd for C 11 h 13 N 2 o 4 S + [M+H] + : 269.0591, found 269.0585.
[0038] The product 3a...
Embodiment 8-11
[0044] A method for preparing diamine derivatives by amide ester, the specific steps are:
[0045] In a 5mL sample bottle, add 145mg 2-acetylaminoethanol acetate 1a, 91.5mg saccharin 2 (the amount of raw material is fixed at 1a:2=2:1), 2mol% hafnium trifluoromethanesulfonate catalyst, and Table 2 medium solvent. Seal and heat to 120°C, and under the condition of constant temperature of 120°C, stir for substitution reaction for 12 hours to prepare diamine derivative 3a, one of the diamine groups of the diamine derivative is retained from the amide ester raw material of carboxylic acid amides.
[0046] Table 2: Experimental parameters for solvent variation
[0047]
[0048] From Examples 8-11, it can be concluded that under the same conditions, the reaction has the highest product yield when no solvent is used. Next, 2 mol% hafnium trifluoromethanesulfonate was used as a catalyst. Under solvent-free conditions, the raw material dosage was fixed at 1a:2=2:1, and the influen...
Embodiment 12-16
[0050] A method for preparing diamine derivatives by amide ester, the specific steps are:
[0051] 145 mg of 2-acetylaminoethanol acetate 1a, 91.5 mg of saccharin 2 (the amount of raw materials was fixed at 1a:2=2:1), and 2 mol% hafnium trifluoromethanesulfonate catalyst were sequentially added into a 5 mL sample bottle. Seal and heat to the temperature in Table 3, and under the condition that the temperature is constant at the temperature in Table 3, stir for a certain period of substitution reaction to prepare diamine derivative 3a, one of the diamine groups of the diamine derivative is reserved Carboxylic acid amides from amide ester raw materials.
[0052] Table 3: Experimental parameters for temperature variation
[0053]
[0054] From Examples 12-16, the optimum reaction temperature for this reaction is 150° C., and the optimum reaction time is 24 hours. Finally, we examine the influence of the substrate and catalyst feed amount changes on the product yield.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com